
Chapter 4: Solvent Recovery/Purging (Rotary Evaporation & Vacuum Purging)
, by Avery Benitez, 39 min reading time

, by Avery Benitez, 39 min reading time

INTRO TO SOLVENT RECOVERY
50L Neocision Rotary Evaporator Turnkey System By BVV
After an initial solvent-based extraction and optional refinement procedures, solvent recovery is necessary to remove solvent from the botanical extract solution. Solvent recovery is by far the most crucial step in creating any solvent-extracted botanical extract. Failure to properly recover an extraction solvent from a botanical extract can result in an unsafe extract for consumption. For this reason, the process of solvent recovery should always be performed extensively and validated by a third-party testing lab to ensure no detectable levels of the solvent remain within the finished extract.
Evaporation is the basic principle behind the solvent recovery process. To evaporate the solvent from a botanical extract, heat must be applied to effectively perform the evaporative process. Evaporation occurs when a compound, in this case, the solvent within a botanical extract, is heated to its boiling point. During this process, once sufficient heat is applied, the botanical extract solution will be observed to boil as the solvent evaporates from the botanical extract solution.
While the process of evaporation is a function of the heat applied to the solution, the efficiency of this process can be significantly increased by pulling the solvent recovery apparatus under a vacuum. When reduced pressure, the form of vacuum is applied to a sealed container, the ambient pressure a compound must overcome in order to evaporate is diminished. This reduction of ambient pressure reduces the resistance a compound must overcome in order to evaporate, which in turn increases the rate of evaporation. The level of heat and vacuum the extract solution is subjected to will determine the rate at which the solvent will be purged from the extract. The more heat, the faster the process, but this may lead to the possible degradation of the botanical extract and the evaporation of the more volatile compounds within the extract.
When it comes to the solvent recovery process, the preservation of lighter volatile compounds within a botanical extract like terpenes should always be secondary to the complete removal of residual solvents. Always aim to apply appropriate heat to fully evaporate the solvent from a botanical extract until no detectable levels remain within the extract. This can easily be achieved by heating the botanical extract solution to the boiling point of the extraction solvent while under vacuum until extract consistency stabilizes and boiling is no longer observed.
A common misconception is that a solvent extracted extract will always have some residual solvent left within it. When solvent recovery is performed properly, all the residual solvent from the extraction process can be effectively purged to Non-detectable levels. After completing solvent recovery of a botanical extract, an extract should never be assumed to be free of residual solvent. The only way to ensure a botanical extract is free of residual solvent is to have the extract tested by an independent third party to ensure its status is free of residual solvents.
The process of removing solvent from a botanical extract can be performed in a variety of different apparatuses ranging from a simple distillation mantel and boiling flasks to more complex rotary evaporators and falling film evaporators. This chapter will cover the most common forms of solvent recovery, rotary evaporation, and vacuum purging utilizing a vacuum oven.
ROTARY EVAPORATORS
50L Neocision Rotary Evaporator Turnkey System By BVV
Rotary evaporators are effective solvent recovery apparatuses that apply basic principles of vacuum distillation to recover solvent from a botanical extract. Rotary Evaporators are used to distill solvents from botanical extract solutions with precise evaporation. Rotary evaporators are especially useful when separating organic compounds from ethanol or any other nonvolatile low boiling point solvents. Rotary evaporators provide increased efficiency over standard mantle-based vacuum distillation apparatuses due to the constant rotation of the boiling flask as it is partially submerged in the heated bath. The constant rotation of the boiling flask creates a thin layer of the solution over the inner surface while evenly heating the outside surface of the boiling flask which results in more efficent heat transfer and increased evaporation rates in comparison to standard mantel-based distillation apparatuses. While rotary evaporators effectively remove the bulk of the solvent from an ethanol extraction, there will still be a trace amount of solvents that can be removed during devolatilization or purged utilizing a vacuum oven or a hot plate inside of an appropriately rated fume hood.
VACUUM PURGING
1.9CF Neocision Vacuum Oven and 4CFM 2 Stage Vacuum Pump Kit By BVV
Vacuum purging is an essential post-processing procedure utilized to remove residual solvent from the botanical extraction process. Vacuum purging is typically performed as the final post-processing step for a botanical extract to remove any residual solvent remaining in the concentrate. Vacuum purging is most effectively achieved utilizing a vacuum oven or a heated vacuum chamber. Vacuum ovens are vessels specifically designed to operate under reduced pressure or vacuum and apply sufficient heat in order to fully recover solvent from a botanical extract. Vacuum ovens use the same basic principles of vacuum distillation in order to evaporate residual solvent remaining in a botanical extract.
Both solvent recovery & vacuum purging are essential in creating a botanical extract free of residual solvent. When it comes to solvent recovery, always aim to remove any and all the solvent from the extraction process to non-detectable levels. An extract should never be assumed to be free of residual solvent. To ensure a botanical extract is free of residual solvent, each batch should always be validated by an independent third-party testing lab. If the testing results show, there are still detectable levels of solvent remaining in the extract, the solvent recovery or vacuum purging process should be continued until testing confirms the extract is free of residual solvent.
BIOMASS SOLVENT RECOVERY
Certified 160L Jacketed Stainless Steel Centrifuge By BVV
After the botanical extraction process, there is often residual solvent left within the spent biomass. To remove this residual solvent, a centrifuge or screw press will be utilized to recover the bulk of the residual solvent with. Often still there is residual amounts of solvent present in the spent biomass which is best treated by a a biomass drying system or incinerator to remove the solvent from the plant matter. Otherwise, biomass with residual solvent is considered dangerous and requires hazardous waste disposal.

Chapter 4: Solvent Recovery/Purging Glossary
Biomass: Generic plant matter used in extraction. Composed of flowers, leaves, and other plant parts.
ROTARY EVAPORATION
50L Neocision Rotary Evaporator Turnkey System By BVV
Rotary evaporation is one of the most common forms of solvent recovery. Also referred to as a rotovap, a rotary evaporator consists of a heated bath and evaporating flask where the evaporative process is initiated, a chilled condenser where the ensuing vapors are recondensed, a receiving flask used to collect and separate the distilled solvent, and a diaphragm vacuum pump used to pull the ensuing vapors through the system and reduce ambient pressure within the system. Optional accessories for a rotary evaporator include a cold trap to recondense any residual vapors before making it to the vacuum pump and a vacuum regulator allowing precise control over the level of vacuum within the system.
Rotary evaporators perform the solvent recovery process by partially submerging the evaporating flask into the heated bath. As heat is applied to the evaporating flask, it is rotated, evenly distributing heat across the entire outer surface of the evaporating flask. The rotation of the evaporating flask creates turbulence within the heated bath, allowing heat to be more evenly distributed within the bath while spreading the internal solution thinly along the inner walls of the evaporating flask which allows vapor bubbles to escape more easily, which in turn increases the evaporation rate of the solution. As the solvent evaporates, a vacuum pump helps lower the ambient pressure within the system and pull the ensuing vapors from the evaporating flask towards the condenser. The condenser contains a coil that is circulated with chilled fluid in order to condense the solvent back into a liquid phase. Once the solvent has been recondensed, it trickles down the condenser coil and collects at the bottom of the condenser into the receiving flask.
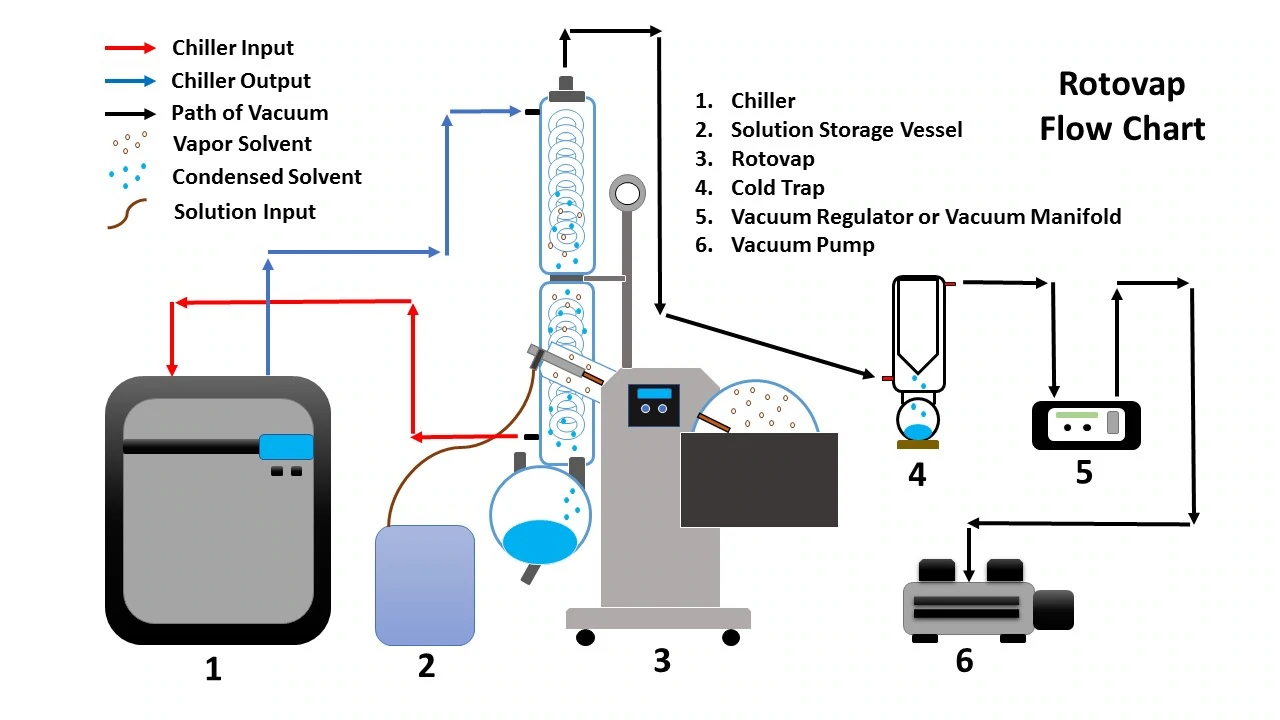
Rotary evaporator operation is a relatively straightforward process. While rotary evaporators function based on several parameters, once these parameters are optimized for the specific process, rotary evaporators carry out the solvent recovery process with minimal interaction from the operator. While a rotary evaporator should never be left to operate independently an operator can monitor operation incrementally while performing other lab tasks ensuring the system is running optimally and the receiving and evaporating flasks don’t overflow.
The process of rotary evaporation starts with the proper assembly of the system, the visual inspection of glass components, and the vacuum testing of the apparatus. After full assembly prior to the operation of a rotary evaporator, it is always best to Inspect the glass components of the rotary evaporator, ensuring all glassware is free of cracks, chips, or defects.
Once the rotary evaporator has been fully assembled and passed the visual inspection prior to performing the solvent recovery process, the entire system should be vacuum tested to ensure the rotary evaporator is properly assembled. A rotary evaporator is vacuum tested by closing all valves on the system except the vacuum valve and turning on the vacuum pump to pull the closed system under vacuum to ensure the desired vacuum levels can be maintained. Vacuum testing helps identify potential vacuum leaks within the apparatus. If desired vacuum levels aren't achieved, proceed to identify where the vacuum leak is coming from by spraying each joint with soapy water. When the vacuum leak is identified, turn off the vacuum pump, open the injection valve allowing the system to reach ambient pressure before disassembling the joint, reapplying vacuum grease to the joint, and reassembling the joint ensuring vacuum grease does not contaminate the inside of the system. Proceed to continue to vacuum test the system again, adjusting each joint as necessary until stable vacuum levels are achieved and maintained.
Once the rotary evaporator has been vacuum tested, proceed to prepare for the solvent evaporation process by bringing the system to operating temperature. To bring the system to operating temperature, first turn on the rotary evaporator and set the heated bath to the desired operating temperature based on the solvent to be removed from the botanical extract. When utilizing higher heated bath temperatures, ensure the heated bath stays at an appropriate fill level by topping off the heated bath with water as needed or switching to a non-toxic heat transfer fluid like silicone oil as the heated bath transfer fluid. Additionally, a rotary evaporator dome cover can be utilized to ensure temperature uniformity and condense heated bath vapors. As the heated bath warms to the desired operating temperature, turn on the condensing coil chiller and set it to 40C/104F colder than the desired heated bath temperature. If utilizing a vacuum cold trap, proceed to bring it to operating temperature by turning on the cold trap chiller to its lowest setting or preparing a dry ice/ethanol slurry.Rotary Evaporator Dome Cover By BVV
As the condensing coil, optional cold trap, and heated bath reach the desired temperature, proceed to elevate the heated bath to partially submerge the evaporating flask. The lip of the heated bath should be no less than 0.5" from the neck of the evaporating flask. Once the evaporating flask has been partially submerged, turn on the rotary function to a low setting and ensure rotation is evenly balanced. If the evaporating flask is rotating unevenly, proceed to turn off the rotary function, lower the heated bath, and allow the evaporating flask to cool before disassembling and readjusting the evaporating flask. Once the evaporating flask has been appropriately readjusted, proceed to partially submerge the evaporating flask, initiate the rotary function, and allow the system to reach the desired operating temperature.
Once the system has reached desired operating temperatures, proceed to turn on the vacuum pump and allow it to reach the desired vacuum levels. Once the desired vacuum level has been achieved, submerge the feed tube into the botanical extract solution and slowly open the feed stopcock to allow a moderate sample of the solution to enter the evaporating flask before closing the feed stop cock.
During this time, it is crucial to monitor the solution and fine-tune the vacuum distillation process to optimize its efficiency before adding more of the solution to the evaporating flask. In order to achieve the most efficient distillation process possible, we will look to optimize the evaporation and condensation of solvent within the rotary evaporator.
To optimize evaporation, we will look to optimize heated bath temperature, evaporating flask rotational speed, and vacuum level. Most commonly, the heated bath temperature will be applied based on the temperature of the solvent the operator is looking to recover. While evaporation of the target solvent can be achieved at lower temperatures with increased vacuum levels. To achieve efficient evaporation of the solvent, it is ideal to heat near the boiling point of the solvent to be recovered.20L Neocision Rotary Evaporator By BVV
Beyond heated bath temperature, the rotational speed of the evaporating flask can also be altered to optimize evaporation. Rotation of the evaporating flask spreads the internal solution thinly along the walls of the evaporating flask, increasing the surface area of the liquid and the rate of evaporation while agitating the liquid in the heated bath, which improves the heat transfer to the flask and subsequent heat transfer to the solution. While most may think faster rotational speed is better, this is not always the case. The evaporation rate can decrease at speeds above a certain point, and high rotational speeds can put unnecessary strain on equipment.
At high rotational speeds, the contents of the flask can be pressed up against the walls of the flask, lowering the turbulence of the liquid and the subsequent evaporation rate. When it comes to rotary evaporation, the speed with maximum turbulence will give the highest evaporation rate. Keep in mind that high turbulence within the evaporating flask usually results in increased agitation and possible spillage of the heated bath, which could pose a potential safety risk. For this reason, rotation speed should be kept at a set point below the point where heated bath spillage occurs.
High Capacity Diaphragm Pump By Welch
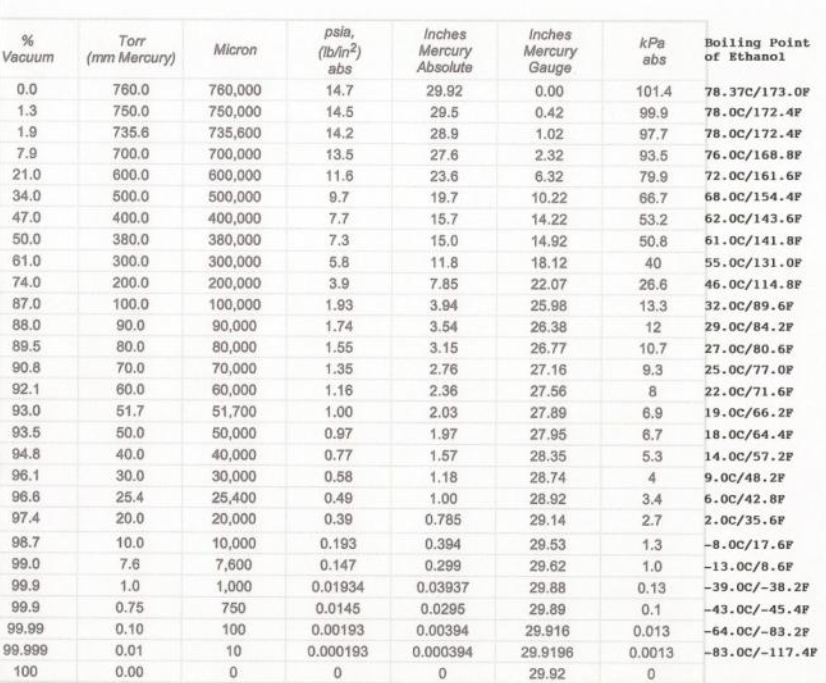
As the solution boils, vacuum level plays a big role in the evaporation rate. When it comes to evaporation, in order for a compound to undergo the phase change from a liquid to a gas, it needs to overcome the atmospheric pressure within the system. Think of atmospheric pressure as an omnipresent weight pushing down at all times. The greater the atmospheric pressure, the more pressure a compound must overcome in order to evaporate, and the more energy in the form of heat is needed for the compound to evaporate from the solution. This concept can be observed when boiling water at different elevations. At high elevations the atmospheric pressure is lower, resulting in water boiling at a lower temperature. At sea level, there is greater atmospheric pressure resulting in water boiling at a hotter temperature.
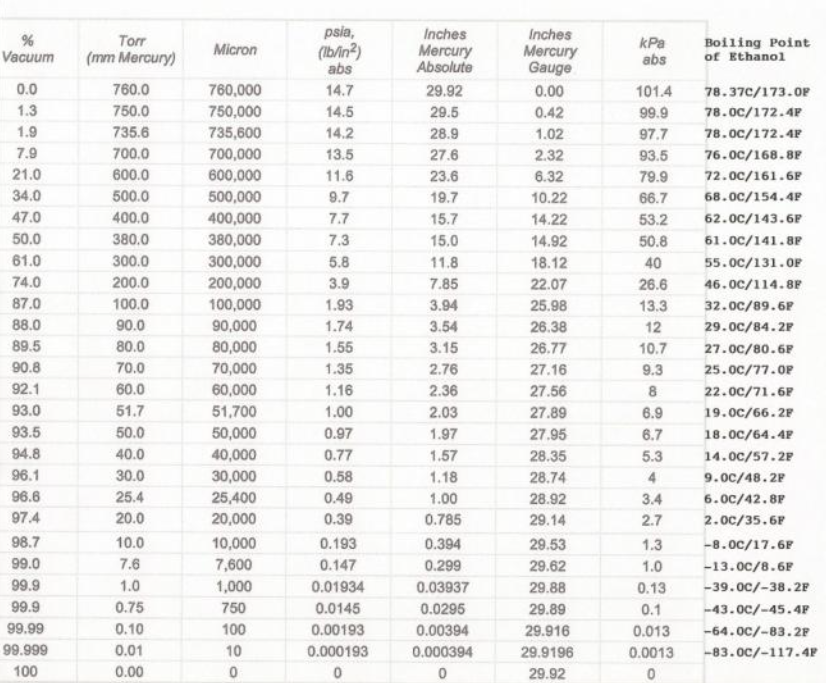
In essence, reduced pressure results in reduced resistance to evaporation, allowing for an increased rate of evaporation. When atmospheric pressure is reduced by pulling the system under vacuum, compounds require less energy in the form of heat in order for evaporation to occur. Therefore, when reduced pressure is applied to a botanical extract solution, the extraction solvent can be evaporated with greater efficiency. By pulling the rotary evaporator under vacuum, not only does the rate of evaporation increase, but it also pulls the ensuing vapors from the evaporating flask through the bump trap/vapor duct into the condenser portion of the rotary evaporator.
DuraChill 1.5HP Chiller By Polyscience
The level of vacuum affects both the rate of evaporation and the pull of vapors through the system. While it is ideal for pulling as deep a vacuum as possible to accelerate the solvent recovery process, vacuum pull should be tuned to the solvent condensing capacity of the condenser coil. The condenser coil is typically chilled to a minimum of 20C/68F cooler than the vapor temperature and 40C/104F cooler than the heated bath temperature to absorb the ensuing vapor's thermal energy, resulting in a phase change from a gas to liquid.
20L Neocision Rotary Evaporator By BVV
During distillation, the condensate level on the condenser coil can be used to optimize the effectiveness of the rotary evaporator. As a general rule of thumb, no more than the bottom 3/4 of the condensing coil should have visible condensate, and there should be no visible condensate on the top quarter of the condenser. If condensate is visible on more than three-quarters of the condenser coil, that is an indication that the rate of evaporation is greater than the rate of condensation. In this case vacuum level or heated bath temperature should be decreased. If less than 3/4 of the condensing coil has visible condensation, that is a good indication that the rate of condensation is greater than the rate of evaporation, and either vacuum or heated bath temperature can be increased in order to accelerate the solvent recovery process.
Vapor Pressure Controller By Digivac
Once the variables of rotary evaporation have been optimized to perform the solvent recovery process, rotary evaporator operation becomes a matter of keeping the solution at the proper level to promote the most efficient solvent recovery and draining the receiving flask to ensure it doesn't overflow into the condenser portion of the rotary evaporator.
During rotary evaporator operation, solution level can be maintained by incrementally adding more of the solution as it evaporates or by throttling the introduction of the feed solution to the rate of the solvent being condensed. While the former is typically the more foolproof option, the latter can be utilized in the hands of an experienced operator for greater efficiency as long as the process is monitored appropriately, ensuring the evaporating flask does not overfill. Regardless of the filling strategy, the evaporating flask fill level should not exceed more than 50% of the total volume of the evaporating flask or be filled above the neck of the evaporating flask to minimize the risk of bumping or foaming above the evaporating flask neck. Keep in mind that too high a liquid level can reduce effective surface area, slowing evaporation. While maintaining too low a fill level can prolong the solvent recovery process.
As the solvent recovery process continues, it is essential to monitor the fill level of the receiving flask as the distilled solvent collects. When the receiving flask is filled near 50% of its total volume the system should be drained by first opening the receiving flask bleeder valve to reduce the vacuum pull on the system and then opening the drain valve of the receiving flask to drain the distilled solvent into an appropriate containment vessel. Once the flask has been completely drained, close the drain valve and the bleeder valve allowing the systems to be pulled back under the set vacuum depth.
Proceed to continue rotary evaporator operation until the entirety of the botanical extract solution has been recovered of solvent and ceases to boil. Once the solvent recovery process visually appears to be complete to ensure the entirety of the solvent has been recovered, proceed to increase the heated bath temperature to 5C and operate the rotary evaporator for another 15-30 minutes to ensure residual solvent is removed. After the solvent recovery process has been completed, the result should be a separation of the target solvent in the receiving flask, and the solvent recovered botanical extract remaining in the evaporating flask. Rotary evaporators are effective in removing the majority of residual solvent from a primary extraction. Depending on the solvent boiling point, it can be a challenge to remediate all residual solvent from a botanical extract with a rotary evaporator alone. Typically with higher boiling point solvents like ethanol, the remainder of residual solvent can be purged from the extract using a vacuum oven or a hot plate under a fume hood.
ROTARY EVAPORATOR STANDARD OPERATION PROCEDURE
Purpose
The purpose of this procedure is to provide detailed instructions for solvent recovery of a botanical extract using a rotary evaporator.
Scope
This procedure applies to all lab technicians tasked with the solvent recovery of a botanical extract.
Definitions/Acronyms
Personal Protection Equipment (PPE) Items worn to protect employees from exposure to hazardous materials and prevention of injury.
Safety Data Sheet (SDS) Provides valuable information on chemicals, describing the hazards the chemical presents, and giving information on handling, storage, and emergency measures in case of an accident.
Safety
SDS Sheets: See extraction solvent SDS for detailed risks
PPE: The following should be worn by all lab personnel while performing solvent recovery of a botanical extract using a rotary evaporator:
Protective eyewear
Lab coat
Gloves
5. Hazard Identification
Preparation and Use:
A botanical extract dissolved in a solvent will be separated by evaporating the solvent from the solution using a rotary evaporator under vacuum.
Concentration- A botanical extract dissolved in a variety of different solvents.
Quantity- no more than 50% of the total volume of the rotary evaporator evaporating flask at a time.
Frequency- Solvent diluted botanical extract is continuously added, evaporated, and condensed until all the solvent has been fully recovered.
Location- Solvent recovery using a rotary evaporator should be performed in a well-ventilated area ideally enclosed within a fume hood.
Potential Hazards and Risks
See extraction solvent SDS for detailed risks.
The explosion from excessive internal pressure: the evaporator flask and/or the condenser could explode if the internal pressure produced by evaporation becomes too great.
Burns: Do not touch the heated bath or evaporating flask during operation.
Rotating hazard: Secure loose clothing, hair, or jewelry. Avoid entanglement of rotating parts which can result in breakage of glassware, burns, and chemical exposure.
Broken glassware: Cracks or chips in glassware can result in glass rupturing causing chemical exposure and explosion hazard to the user. Always inspect glassware prior to use.
6. Preparation
1. Inspect and fully assemble the rotary evaporator ensuring all glassware is free of cracks, chips, or defects.
2. Vacuum test the system by closing all inlet and outlet valves on the rotary evaporator and turning on the vacuum pump allowing the entire rotary evaporator to be pulled under vacuum. If desired vacuum levels aren't achieved proceed to identify where the vacuum leak is coming from by spraying glass joints with soapy water. When the leak is identified disassemble and reassemble the joint applying appropriate vacuum grease to the joint ensuring vacuum grease does not contaminate the inside of the system. Apply vacuum again, If a strong vacuum is achieved proceed to prepare for solvent evaporation by bringing the system to operating temperature
3. Turn on the heating bath and set the temperature to the boiling point of the solvent you are looking to recover.
4. Turn on the condensing coil chiller setting it to a minimum 40C/104F lower than the heated bath setting (Or as cold as your condenser chiller permits) if using a cold trap proceed to turn on the cold trap chiller to its lowest setting or prepare a dry ice slurry.
5. Raise the heated bath to partially submerge the evaporating flask. Ensure there is a minimum of 0.5" clearance between the evaporating flask and the edge and or bottom of the heating bath.
6. Turn on the rotary function setting the speed to 20-50 RPM assuring the rotation is stable. If the evaporating flask is rotating unevenly proceed to turn off the rotary function, lower the heated bath, and allow the evaporating flask to cool before disassembling and readjusting the evaporating flask. Once the evaporating flask has been appropriately readjusted proceed to partially submerge the evaporating flask, initiate the rotary function and allow the system to reach the desired operating temperature.
7. Place feed tube into the botanical extract/solvent solution.
6. Procedure
Slowly open the feed stopcock to allow the solution to enter into the evaporating flask. Start with a small volume to fine-tune the evaporator process and add more once evaporation is optimized. Once the desired fill level has been achieved close the feed stopcock.
During operation alter the system parameters to optimize the distillation of solvent.
Fine-tune rotational speed to maximize turbulence within the evaporating flask without causing heated bath spillage.
Regulate vacuum level to optimize the performance of the condenser by increasing or decreasing vacuum level so no more than 3/4 of the condenser has visible condensate. The top 1/4 of the condenser coil should not have any visible condensation to ensure all volatiles are being condensed before exiting the rotary evaporator.
Regulate evaporating flask solution volume to optimize the turbulence of the liquid (Never exceed 50% of the total volume of the rotary evaporator evaporating flask).
If the solution is ever boiling too vigorously or causing bumping into the neck of the bump trap, first decrease vacuum, lower the heated bath, reduce rotational speed and decrease the heated bath temperature before reinitiating the solvent recovery process.
Allow the solution to evaporate until boiling has subsided completely and all volatiles have been removed.
As the receiving flask fills with solvent, to drain the distilled solvent first release vacuum from the flask by closing the backflow valve between the condenser and receiving flask, and open the receiving flask drain valve to and drain the distilled solvent from receiving flask into an appropriate vessel.
Close drain and vent valve on receiving flask and allow the system to equalize back to the desired vacuum level.
When the solution consistency stabilizes and no more boiling is observed, decrease rotational speed, increase heated bath temperature by 5C/41F, and increase vacuum depth if possible, allowing the flask to remain at reduced pressure, while heating, and rotating for at least 15 minutes until no more bubbling is observed. Once the bubbling subsides turn off rotation and allow the extract to sit stationary for 20-30 minutes before proceeding to shut down the system and harvest the extract to ensure all of the volatiles are removed.
Once the evaporation is complete proceed to shut down the rotary evaporator by turning off the rotation of the evaporating flask, lowering and turning off the heated bath, closing the vacuum valve, turning off the vacuum pump and, opening the stopcock to pressurize the system to ambient pressure. Once the system has been brought to ambient pressure turn off the condenser and cold trap chiller and allow the evaporating flask to cool before attempting to remove the evaporating flask and drain the contents into a clean receiving vessel.

VACUUM PURGING
1.9CF Neocision Vacuum Oven By BVV
Vacuum purging is an essential post-processing procedure utilized to remove residual solvent from the botanical extraction process. Vacuum purging is typically performed as the final post-processing step to ensure the extract is safe for consumption or infusion. The vacuum purging process is essential in creating a safe botanical extract—failure to fully recover the initial extraction solvent results in a botanical extract that is unsafe for consumption. Therefore, great care should be observed to thoroughly remove any solvent from the extraction process to non-detectable levels. An extract should never be assumed to be free of residual solvent. In order to ensure a botanical extract is free of residual solvent, each batch should always be validated by an independent third-party testing lab. If the testing results show there are still detectable levels of solvent remaining in the extract, the vacuum purging process should be continued at a higher temperature or a longer duration until testing confirms the extract is free of residual solvent.
CVO-2 Vacuum Oven By Cascade Sciences
The process of removing residual solvent from a botanical extract is most effectively achieved through the use of a vacuum chamber or a vacuum oven. Both vacuum chambers and ovens utilize reduced pressure to purge a botanical extract of residual solvent effectively. Vacuum chambers and ovens are essentially air-tight vessels designed to operate under reduced pressure.
Reduced pressure is applied to the vessel through a spark-free vacuum pump. A vacuum pump removes gas molecules and air particles from a sealed vessel reducing pressure until a full vacuum is achieved. A vacuum is essentially a space devoid of matter where ambient pressure inside the vessel is below atmospheric. When a closed container is pulled under a vacuum, it reduces pressure. When pressure is reduced within a vessel, the resistance a compound must overcome in order to evaporate is reduced.
V4D 4CFM Two Stage Vacuum Pump By BVV
When it comes to evaporation, for a compound to undergo the phase change from a liquid to a gas, it needs to overcome the ambient pressure within the system. Think of ambient pressure as an omnipresent weight pushing down at all times. The greater the atmospheric pressure, the more energy in the form of heat is needed for compounds to evaporate. This can be observed when boiling water at different elevations. There is less atmospheric pressure at high elevations, resulting in water being observed to boil at a lower temperature. At sea level, the weight of the atmosphere is greater, resulting in more heat needing to be applied in order for the water to overcome the weight of the atmosphere and evaporate.
In essence, reduced pressure reduces resistance to evaporation, allowing for the evaporation of compounds at lower temperatures. When atmospheric pressure is reduced, compounds require less energy in the form of heat in order for evaporation to occur. Therefore when reduced pressure is applied to a botanical extract, it can be purged of residual solvent, more effectively resulting in reduced heat exposure and limited degradation of the botanical extract.
Reduced pressure/vacuum increases the efficiency of the evaporative process to purge all the residual solvent from an extract effectively. Heat near the equivalent of the target compound's boiling point must be applied in conjunction with vacuum to recover the solvent from an extract to non-detectable levels effectively. While vacuum alone can be utilized to purge solvent to some extent to reach the ideal non-detectable level of solvent, heat near the boiling point of the target compound must be applied.
This can become a challenge when looking to preserve highly volatile compounds within a botanical extract during the vacuum purging process, even though it may seem logical to achieve this by simply applying a lower heat setting for a longer duration of total time. We recommend a quick and efficient purge using sufficient heat and vacuum to reduce the amount of time the extract is subjected to heat and vacuum. A longer vacuum purge allows more time for the desired compounds to evaporate. By applying sufficient heat and vacuum for a shorter duration, more volatile compounds can be preserved within the botanical extract by limiting the extract's exposure to heat and vacuum.
Even at the higher temperature spectrum, vacuum purging is a relatively slow process. It takes time to purge a botanical extract of residual solvent effectively. The amount of time it takes to purge residual solvent is dependent on the amount of vacuum and temperature applied to the extract. It is not uncommon for the vacuum purging process to take multiple hours or even days to purge all residual solvents effectively. While the only way to ensure an extract is free of residual solvent is through lab testing. The process is most effectively gauged visually by the rate of boiling. When boiling is no longer observed and extract consistency stabilizes, it is typically an indication that the majority of the residual solvent has been purged. Once boiling has completely subsided, it is best to continue the process at a slightly higher temperature under full vacuum for 1-2 more hours to ensure all solvent has been purged before sending a sample of the extract for third-party analysis.
10" Cold Trap With KF25 Ports By BVV
Prior to performing the vacuum purging process, it is important to install the vacuum oven or chamber in an area with adequate ventilation where it won't be exposed to combustible or flammable gases. Vacuum ovens are not designed for use in hazardous locations where they will be exposed to outside combustible or flammable gases. Vacuum ovens should only be used in areas for which their electrical components are properly rated, ideally under a fume hood. The outgassed byproducts may be hazardous for operating personnel during the vacuum purging process. For this reason, it is highly recommended that a cold trap be used to condense the ensuing vapors prior to them reaching the vacuum pump. Additionally, the vacuum pump exhaust should be ducted outside the facility to ensure solvent vapor is not recycled back into the lab.

NEOCISION Cold Trap - ETL Rated - (-80c) By BVV
After the vacuum oven has been fully assembled with the vacuum pump exhaust ducted outside the lab, vacuum purging preparation starts with the chilling of the cold trap and the preparation of the botanical extract. Prior to performing the vacuum purging process, proceed to chill the cold trap utilizing a dry ice ethanol slurry or an appropriate immersion probe and prepare the botanical extract before loading it into the vacuum oven. Before loading the extract into the vacuum oven, it is best to first spread the botanical extract thinly on a piece of parchment paper, PTFE sheet, or Pyrex dish. Thinly spreading the extract increases its surface area and allows solvent to boil from the extract more easily. Once the extract has been thinly spread, it can then be loaded into the center of the vacuum oven. Once the extract has been loaded into the vacuum oven, close the vacuum oven door and ensure the outlet port is open so pressure doesn't build within the oven while the vacuum oven & botanical extract are warmed to operating temperature.
Roll of Oil Slick PTFE Sheet By Oil Slick
Now that the extract has been loaded into the vacuum oven with the vacuum oven door closed and the outlet port open, allow both the extract and vacuum oven to reach the desired temperature setting before applying vacuum by turning on the vacuum oven and setting the temperature to the desired heat setting. When selecting a vacuum purging temperature, keep in mind the boiling point of the solvent to be purged and the boiling point of the desired compounds in the botanical extract to be preserved. While the vacuum purging process can be performed using lower temperatures for a longer duration, the vacuum purging process is most efficiently performed using sufficient heat and vacuum. A temperature setting between 80F -110F is commonly utilized to recover low boiling point solvent from a botanical extract depending on the type of extract and solvent being recovered.
1.9CF Neocision Vacuum Oven By BVV
As the vacuum oven and botanical extract are brought to the desired temperature, prepare to apply vacuum to the vessel by allowing the spark-free vacuum pump to warm up for 10-15 minutes before applying vacuum to the system. First, ensure the vacuum oven vacuum port is closed before turning the vacuum pump on and allowing it to warm up. Once the vacuum oven has reached the desired temperature and the vacuum pump has warmed up, proceed to slowly pull the system under vacuum by slowly opening the pump hole switch allowing the system to be pulled under light vacuum. During this time, closely monitor the extract's rate of boiling to ensure the extract does not boil out of its container resulting in loss of yield and contamination of the vacuum oven. As boiling reduces, slowly apply more and more vacuum in -5Hg increments keeping a close eye on the botanical extract to prevent over-boiling. If an extract looks like it is overboiling, reduce vacuum by closing the pump inlet switch or opening the air inlet switch to bleed atmosphere into the vacuum oven. If the extract is boiling in a controlled manner, continue to increase vacuum until a full vacuum is applied.
Over time, as the amount of residual solvent within the extract decreases, boiling lessens. Eventually, all boiling will cease, the texture and consistency of the extract equalize, and the vacuum purging process will visually appear to be complete. This can take hours or days depending on vacuum level, purge temperature, the solvent used, and botanical extract type. When boiling appears to subside completely, it is recommended to increase the temperature slightly and let the extract sit for 1-2 more hours to ensure all residual solvent has been purged prior to sending a sample out for independent third-party testing.
After the vacuum purging process is complete, close the vacuum pump port, turn off the vacuum pump, and depressurize the system by slowly opening the outlet port. The outlet port should be opened slowly not to disturb the contents of the vacuum oven. Once the system pressure has been equalized to ambient pressure, open the vacuum oven door and collect the extract.

VACUUM PURGING STANDARD OPERATING PROCEDURE
Purpose
The purpose of this procedure is to provide detailed instructions for solvent recovery of a botanical extract using a vacuum oven.
Scope
This procedure applies to all lab technicians tasked with the solvent recovery of a botanical extract.
Definitions/Acronyms
Personal Protection Equipment (PPE) Items worn to protect employees from exposure to hazardous materials and prevention of injury.
Safety Data Sheet (SDS) Provides useful information on chemicals, describing the hazards the chemical presents, and giving information on handling, storage, and emergency measures in case of an accident.
Safety
SDS Sheets: Extraction solvent SDS for detailed risks
PPE: The following should be worn by all lab personnel while performing solvent recovery of a botanical extract using a vacuum oven:
Protective eyewear
Lab coat
Gloves
5. Hazard Identification
Preparation and Use:
A botanical extract will be purged of residual solvent using reduced pressure and heat utilizing a vacuum oven in conjunction with a vacuum pump and cold trap.
Concentration- Residual solvent remaining within a botanical extract.
Quantity- no more than 5% of the total extract volume by weight.
Frequency- The botanical extract contains residual solvent; the solvent is evaporated from the extract until it's fully purged, no more solvent is added.
Location- Solvent recovery using a vacuum oven should be performed in a well-ventilated area with vacuum pump exhaust ducted outside of the facility.
Potential Hazards and Risks
See extraction solvent SDS for detailed risks.
Vacuum ovens are heated; direct contact with heated surfaces may cause irritation or burns.
Vacuum ovens are not rated for pressure and will open when pressurized, potentially exposing operators to heat and potentially harmful vapors.
Vacuum ovens are not designed for use in hazardous locations where they will be exposed to combustible or flammable gases. Vacuum ovens should only be used in areas for which their electrical components are properly rated.
6. Preparation
1. Position the vacuum oven, cold trap, and vacuum pump in a permanent location where no combustible or flammable gases are present and exhaust gases can be easily ducted outside the facility.
2. Connect the vacuum oven to the cold trap and spark-free vacuum pump utilizing appropriate vacuum hoses. Ensure no hoses are kinked, bent, or positioned in a way that bears weight. Hoses should hang comfortably from their fittings.
3. Vacuum test the oven, pump, and cold trap in their final installed location by turning on the vacuum pump and allowing the entire system to be pulled under vacuum. If the desired vacuum level isn't maintained proceed to check all process connections and seals disassembling and reassembling the system as needed until vacuum levels are maintained. Once vacuum levels are maintained proceed to turn off the vacuum pump, depressurize the system and proceed to the vacuum oven operation procedure.
6. Procedure
Chill the cold trap to the desired operating temperature using an appropriate chiller or dry ice/ethanol slurry.
Place your botanical extract on a piece of parchment paper, PTFE sheet, or a Pyrex dish.
Spread the extract thinly to ease the evaporation of the residual solvent.
Load the botanical extract into the vacuum oven and close the vacuum oven door.
Orient the inlet switch, pump hole switch, in their closed positions and ensure the outlet hole is in its open position as the extract and oven are warmed to operating temperature.
Turn on the vacuum oven power switch and set the oven to your desired internal temperature.
Ensure the vacuum outlet is closed before turning on the vacuum pump and allowing it to warm up for 10-15 minutes as the vacuum oven reaches operating temperature.
Once the vacuum oven has reached the desired operating temperature and the vacuum pump has been adequately warmed up, slowly open the vacuum valve to start pulling the chamber under vacuum in -5Hg increments.
Monitor the extract closely if excessive boiling is observed reduce vacuum pull by closing the vacuum valve or opening the outlet hole.
If the extract is boiling in a controlled manner proceed to increase vacuum depth by -5Hg while monitoring the extract closely to avoid overboiling. Once the desired vacuum level has been achieved continue vacuum oven operation until boiling is no longer observed.
Once extract consistency stabilizes and boiling is no longer observed continue the vacuum purging process at a slightly higher heat setting for 1-2 more hours to ensure residual solvent has been effectively purged.
If no more boiling is observed the vacuum purging procedure is complete. To shut down the vacuum oven operation first close the vacuum valve, turn off the vacuum pump, and slowly open the outlet hole to bring the vacuum oven to atmospheric pressure.
If confident the extract is purged of all residual solvent, send a sample to be analyzed by an independent third-party lab to ensure no solvent remains in the extract. If the test shows the botanical extract still contains residual solvent resume the vacuum purging process at an even higher temperature until extract consistency stabilizes and boiling is no longer observed and proceed to test the extract again until no detectable levels of residual solvents remain in the extract.
Once the extract has passed its residual solvents test proceed to final packaging or further post-processing.