
Chapter 1: Intro to Botanical Extraction & Refinement
, by Avery Benitez, 45 min reading time

, by Avery Benitez, 45 min reading time

Botanical extraction is a fascinating process used widely to create some of the modern products used daily. From medicine to cleaning agents, powerful botanical compounds can be extracted from a wide variety of different plants. In this section, we will introduce the different methods of solvent-based botanical extraction along with some of the most common post-processing methods of refinement used to create botanical extracts.
Currently, there are several popularized methods of botanical extraction in today's extraction landscape, all of which have their own use cases and unique benefits. Most botanical extraction methods can be categorized into two different methodologies, either solvent-based extraction or solventless-based extraction. A solvent-based botanical extraction method utilizes organic solvents to dissolve target compounds from the biomass material. Among the solvent-based extraction methods, hydrocarbons and ethanol are the most commonly used for botanical extraction and will be the focus of this course.
5LB EVO Certified Closed Loop Extractor 49 State Certified by BVV
Hydrocarbon extraction is a widely used botanical extraction method that utilizes light hydrocarbon solvents like butane and propane to extract botanical compounds. Hydrocarbon extraction is preferred by many botanical extractors due to its high yields and low solvent recovery temperatures. These highly versatile extraction solvents lend themselves to producing a high purity botanical concentrate with little to no post-processing. Hydrocarbons are a classification of organic compounds made from carbon and hydrogen. These hydrocarbons are formed due to the compression of animal and plant remains over long periods and are pulled from porous rocks where they pool and concentrate. These alkane molecules have an even distribution of electrons, making them nonpolar and well suited for extracting nonpolar botanical compounds.
The most commonly used hydrocarbons for botanical extraction include butane and propane. These hydrocarbons can be utilized on their own to perform botanical extraction, or they can be combined into a blend of hydrocarbon solvents and utilized to extract a full spectrum of botanical compounds from biomass material. Typically a blend of n-butane, Isobutane, and propane is utilized to create an extraction solvent with greater affinity to extract a wider variety of compounds found within the biomass material resulting in a more full-spectrum extract.20LB High Purity N-Butane By BVV
Both butane and propane are class 1 group D flammable liquified gases that the Food and Drug Administration (FDA) have generally recognized as safe (GRAS). These light hydrocarbons are gaseous at room temperature and ambient pressure, where they are denser than air and tend to pool near the floor. When pressurized or placed under reduced temperature, they become liquified and can be utilized to extract nonpolar botanical compounds from biomass material.
Hydrocarbons are the preferred extraction solvent of many extractors looking to preserve highly volatile aromatic compounds due to their low boiling point -1C/30.2F for butane and -42C/-43.6F for propane. Since these solvents boil off at a lower temperature than most aromatic compounds, they can be recovered with minimal heat preserving more aromatic compounds in comparison to other high boiling point extraction solvents like ethanol. While butane and propane's low boiling points lend themselves to volatile compound preservation, it also makes hydrocarbons highly volatile and more hazardous to work with, requiring greater safety requirements.
Although butane is generally recognized as safe by the FDA and can be consumed by humans in trace amounts, utilizing these highly volatile and flammable hydrocarbons as organic solvents under extreme temperatures and pressures can be dangerous. While it is highly unlikely that hydrocarbon extraction will result in an explosion when performed properly, when utilizing these highly flammable gases, there is always an inherent potential for ignition. For this reason, any and all potential sources of heat or flame should be kept far away from areas in which hydrocarbon gases are present. To further mitigate the potential for ignition, hydrocarbon solvent extraction should be performed inside a C1D1 explosion-proof enclosure with high air exchange rates and all the proper safety and protective equipment.45L Jacketed Stainless Steel centrifuge By BVV
Ethanol extraction is an excellent method of botanical extraction valued for its high throughput potential and reduced flammability compared to more volatile solvent-based extraction methods like hydrocarbon extraction. Ethanol is classified as alcohol made from starch-based plant materials through a complex fermentation and distillation process. Ethanol (EtOH), commonly known as ethyl alcohol or grain alcohol, is a colorless flammable liquid with an incredible number of uses beyond botanical extraction, including fuel and a cleaning agent.
As an extraction solvent, ethanol is unique due to its amphiphilic nature, meaning it has both polar and nonpolar properties. Ethanol has a hydrotropic polar head (water-loving) that can bind to water-soluble compounds, and a nonpolar tail that can dissolve hydrophobic or oil-soluble compounds typically found in botanical biomass. As the plant matter is saturated with ethanol, it dissolves both polar and nonpolar compounds from the biomass material, extracting them. Ethanol's amphiphilic nature can be ideal when seeking to create a full-spectrum product, but it can be less than ideal for those looking to create a more concentrated extract.270 Gallon Tote of 200 Proof Food & Lab Grade Corn-based Ethanol By BVV
Due to ethanols' polar and nonpolar nature, it has an increased propensity to co-extract undesirable plant waxes and chlorophyll during the extraction process. This typically results in a less pure end product requiring more post-processing to reach high levels of purity. While ethanol’s selectivity for undesirable plant waxes and chlorophyll can be altered by chilling the ethanol prior to extraction, reducing the solubility of undesirable plant waxes and color pigments, it results in reduced solubility of target compounds as well.
Beyond its polarity, ethanol has a high saturation capacity and only requires a short retention time to dissolve target compounds lending itself to short runtimes and high throughput potential. Ethanol is particularly well suited for bulk processing, with available systems that can extract thousands of pounds of biomass within a single working shift. This makes ethanol a great option for those making botanical extracts at larger scales.
During the botanical extraction process, it is common that some undesirable plant compounds will be co-extracted alongside the target compounds. These compounds typically include plant waxes, fats, lipids, and color compounds, including chlorophyll, anthocyanins, and flavonoids. The co-extraction of these undesirable compounds produces an extract that is typically lower in the concentration of target compounds and visually unappealing.
Luckily several post-processing procedures can remove these undesirable compounds and create a highly refined and pure botanical extract. These processes after the initial extraction are classified as post-processing methods. Typically to produce a high-quality refined botanical extract from solvent-based extraction methods, most crude botanical extracts will be refined by removing excess undesirables through winterization and color remediation, purging of residual solvent from the extraction process, the devolatilization and distillation of the extract, and finally, the isolation of target compounds from the refined distillate. These individual processes encompass the entirety of botanical extract refinement and will be covered in depth in the post-processing chapters of this course.
Explosion Proof Dual Temp Freezer By So-Low
Winterization is a widely used refinement technique to remove plant waxes, fats, and lipids from a botanical extract. Winterization is often seen as a required post-processing procedure for most solvent-based extractions as these plant lipids are typically dissolved to some extent during a solvent-based extraction. While the pickup of plant waxes can be limited by altering the temperature and saturation time of the initial extraction, it is commonly unavoidable to co-extract a small amount of these compounds during a solvent-based extraction. The process of removing these plant waxes from a botanical extract is most efficiently performed through winterization. Winterization creates a more refined botanical extract resulting in greater purity and transparency.
Winterization utilizes the principle of solubility to precipitate plant fats, waxes, and lipids from a botanical extract solution. In order to perform winterization first, a botanical extract will need to be dissolved in a solvent that the plant waxes are soluble in when heated but have low solubility in that same solvent when chilled. This difference in plant wax solubility at high and low temperatures allows us to completely dissolve the plant waxes into the solution at high temperatures and precipitate the plant waxes from the solution at low temperatures. Once the fats and lipids have consolidated, they are cold-filtered, removing fats, waxes, lipids from crude oil before further refinement. When this process is complete, the result is a refined extract referred to as a winterized extract.
6" Color Remediation Column By BVV
Color remediation is a powerful refinement process that is most commonly achieved through inline adsorbent filtration. Adsorbent filtration involves the use of adsorbent media to separate the compounds of a chemical mixture based on the interaction of the adsorbate with the adsorbent. In this case, the adsorbates are the color compounds being adsorbed, and the adsorbent is the filtration media adsorbing the color compounds. Adsorption is not to be confused with absorption, which is when fluid is dissolved by or permeates a liquid or solid. Adsorption refers to the adhesion of atoms, ions, or molecules from a gas, liquid, or dissolved solid to a surface.
In this process, a nonpolar botanical extract solution passes through a bed of adsorbent filtration media. Depending on the type of media used, certain compounds are adsorbed from the solution. The adsorption process creates a film of the adsorbate on the surface of the adsorbent, holding the adsorbate back while allowing the rest of the solution to flow through the media. Adsorbents are generally porous in nature, with a high surface area to adsorb substances to their surface. Color remediation greatly increases the purity of the resulting botanical extraction, providing a visually more appealing and pure botanical extract.
Falling Film Evaporator By BVV
After an initial solvent-based extraction and optional refinement procedures, the process of solvent recovery is necessary to remove solvent from the botanical extract solution. Solvent recovery is by far the most crucial step in creating any solvent-extracted botanical product. Failure to properly recover an extraction solvent from a botanical extract can result in an extract unsafe for consumption. For this reason, the process of solvent recovery should always be performed extensively and validated by a third-party testing lab to ensure no detectable levels of the solvent remain within the finished extract.
Evaporation is the basic principle behind the solvent recovery process. Heat must be applied to trigger the evaporative process from a botanical extract. Evaporation occurs when a compound, in this case, the solvent within a botanical extract, is heated to its boiling point. Once sufficient heat is applied during this process, the botanical extract solution will be observed to boil as the solvent evaporates from the botanical extract solution. Once the solvent has been evaporated from the solution, it is typically recondensed back into a liquid separate from the botanical extract allowing the solvent to be reused in most cases.
The process of removing solvent from a botanical extract can be performed in a variety of different apparatuses ranging from a simple distillation mantle and boiling flasks to more complex rotary evaporators and falling film evaporators. Regardless of the apparatus, the solvent recovery process is most efficiently performed by applying both heat and vacuum. When a solvent recovery apparatus is pulled under vacuum, it reduces the ambient pressure within the vessel, reducing the amount of pressure a compound must overcome to evaporate. By applying vacuum to the solvent recovery procedure, the resistance to evaporation can be greatly reduced, drastically increasing the overall efficiency of the process. This course will cover the most common forms of solvent recovery for a botanical extract, rotary evaporation, and vacuum purging utilizing a vacuum oven.
50L Neocision Rotary Evaporator Turnkey System By BVV
Rotary evaporators are effective solvent recovery apparatuses that apply basic principles of vacuum distillation to recover solvent from a botanical extract. Rotary Evaporators are used to distill solvents from botanical extract solutions with precise evaporation. Rotary evaporators are especially useful when separating organic compounds from ethanol or any other nonvolatile low boiling point solvents. Rotary evaporators provide increased efficiency over standard mantle-based vacuum distillation apparatuses due to the constant rotation of the boiling flask as it is partially submerged in the heated bath. The constant rotation of the boiling flask creates a thin layer of the solution over the inner surface while evenly heating the outside surface of the boiling flask resulting in increased evaporation rates compared to standard mantle-based distillation apparatuses.
1.9CF Neocision Vacuum Oven By BVV
Vacuum purging is an essential post-processing procedure that removes the residual solvent from the botanical extraction process. Vacuum purging is typically performed as the final post-processing step for a botanical extract to remove any residual solvent remaining in the concentrate. Vacuum purging is most effectively achieved utilizing a vacuum oven. Vacuum ovens are vessels specifically designed to operate under reduced pressure or vacuum and apply sufficient heat to fully recover solvent from a botanical extract. Vacuum ovens apply the same basic principles of vacuum distillation to evaporate residual solvent remaining in a botanical extract.
Both solvent recovery & vacuum purging are essential in creating a botanical extract free of residual solvent. When it comes to solvent recovery, you should always aim to remove any or all the solvent from the extraction process to non-detectable levels. An extract should never be assumed to be free of residual solvent. An independent third-party testing lab should always validate each batch to ensure a botanical is free of residual solvent. If the testing results show there are still detectable levels of solvent remaining in the extract, the solvent recovery or vacuum purging process should be continued at a slightly higher temperature or a longer duration until testing confirms the extract is free of residual solvent.
20L Double Jacket Glass Reactor By BVV
Devolatilization is often defined as the removal of volatile compounds within a substance. This is often necessary to further refine a botanical extract via distillation. Devolatilization is an evaporative process that drives volatiles from a botanical extract when heat is applied. Typically this is a separate process performed before distillation to ensure the protection and performance of mechanical systems during distillation.
One of the more common forms of devolatilization of a botanical extract is decarboxylation. Decarboxylation is a chemical reaction that removes a carboxyl group from an organic compound releasing carbon dioxide. Typically decarboxylation is performed to remove loosely bonded carboxylic acids from the atomic chain of a botanical compound, converting it into a more stable, easily distilled form. Decarboxylation is commonly used to convert acidic phytocannabinoids into their non-acidic active forms by evaporating the loosely bonded carboxyl group from the compound, releasing CO2. Decarboxylation of phytocannabinoids should always be performed before distillation as the release of CO2 negatively affects vacuum pump performance.
Devolatilization as a process is used to remove volatile compounds from a botanical extract, convert compounds into a more distillable form, and ensure an extract is effectively purged of residual solvent prior to performing distillation. When a botanical extract is properly devolatilized, it results in a smoother, more efficient distillation of a botanical extract.
5L Neocision Short Path Distillation Turnkey Kit By BVV
Distillation is a powerful purification technique that can drastically increase the purity of a botanical extract. Distillation can be simplified as the act of purifying a liquid by using selective evaporation and condensation. This purification process is implemented by heating the input material until target compounds are evaporated and then pulling the resulting vapor through a chilled condenser where it is condensed back into liquid form and collected. Distillation carries excellent utility in separating compounds in a mixture when there is a significant enough difference between their boiling points and or their vapor pressure.
Distillation works by applying a gradual temperature increase to refined botanical extract while under vacuum to evaporate the target compounds from the crude oil. As the boiling points of the target compounds have been reached, they are evaporated and carried via vacuum to a cooled condenser to recondense the target compounds into a separate vessel. This process of evaporation and condensation creates an extremely pure botanical concentrate referred to as distillate.
Certified Jacketed Diamond Miner By BVV
Isolation represents the final refinement step for botanical extract resulting in a highly refined concentrate of the target compound reaching upwards of 99% purity. While Isolation of target compounds can be achieved via fractional distillation or chromatography, the focus of this section will be on crystallization as means of Isolation. Crystallization is a purification technique used to isolate crystalline compounds from a solution. This is achieved by manipulating the solubility of the target compound when dissolved in a crystallization solvent. Crystallization is most commonly achieved through the evaporation of solvent or the cooling of the solution to achieve supersaturation of the target compound within the solution.
Crystallization utilizes the concept that different compounds have different solubilities within the same solvent and that only molecules of the same crystalline compound will align into the lattice structure of the crystal formation. During crystallization, impurities can only stick to the outside of the crystal lattice or remain within the solution. In practice, crystallization of a target compound is performed by achieving supersaturation through evaporation of the solvent from the solution or cooling of a solution to reduce its solubility to the point of supersaturation. When a solution becomes supersaturated, it contains more of the solute than the solvent can hold. Crystalline compound clusters align within the solution resulting in a crystalline structure precipitating out of the solution. This process of precipitating a crystalline compound out of a solution is referred to as crystallization.

The following glossary compiles words and terminology used throughout this course. These definitions are meant to provide the reader a quick definition of terms without being too exhaustive. Refer back to this section if you need further clarification of a used term.
Absorption: The process or action by which one thing absorbs or is absorbed by another.

When performing botanical extraction, safety should always be the number one concern. The key to safely performing botanical extraction lies in handling hazards appropriately by operating within a controlled environment, wearing the proper personal protective equipment, and utilizing clearly defined operating procedures.
When dealing with flammable solvents during the botanical extraction and refinement process, there is always an inherent hazard level. While varying degrees of hazard are ever-present during the botanical extraction process, when these hazards are properly managed and controlled, the botanical extraction process can be performed with minimal risk to the operator or the surrounding areas.
During the botanical extraction process, hazards can range from flammable solvents, toxic chemicals, inhalation hazards, and equipment under pressure, vacuum, or extreme temperatures. A hazard is defined as a potential source of harm. Substances, events, or circumstances can constitute hazards when their nature would allow them, even just theoretically, to cause damage to health, life, property, or any other interest of value.
Potential hazards are commonly ever-present during the botanical extraction process. While we can limit potential hazards through environmental control, clearly defined standard operating procedures, employee training, and personal protective equipment, accidents happen. Before accidents happen within a botanical extraction, lab it is crucial to be prepared by ensuring the botanical extraction lab has easily accessible emergency eyewash stations, chemical showers, appropriately rated fire extinguishers, and safety data sheets for all used hazardous materials available and easily accessible at all times.
Combination Shower & Eyewash Station By Guardian Equipment
The extraction solvent often presents the greatest chemical hazard in many botanical extraction facilities. With hydrocarbons and alcohols being highly flammable, it is important to store all solvents in an approved fire-safe solvent cabinet and operate solvent-based extraction techniques enclosed within an appropriately rated hazardous location based on the local, state, and federal requirements.
When storing any type of hazardous materials the safety data sheets (SDSs shall be readily accessible during each work shift to employees when they are in their work areas. Containers and/or packages related to hazardous materials must be properly labeled and warning signage should be properly displayed and easily visible. Ensure that all primary containers (i.e. original container in which a chemical arrives from the supplier) and secondary containers (beaker, bottle, etc.) are affixed with an OSHA-compliant/GHS label. All personnel should be properly trained on OSHA's hazard communication (Right to Know) standard including, but not limited to: operations in their work area where hazardous chemicals are present; methods that may be used to detect the presence or release of a hazardous chemical in the work area: measures employees can take to protect themselves from these hazards: details of the hazard communication program; chemical labeling: and location of SDSs and trained on what to do in the event of an emergency involving any of the hazardous material on the property. Staff monitoring the extraction process must be trained on the extraction process, the transfer of solvents, and all emergency procedures. Record of staff training must be maintained on-site and made available to the authority having jurisdiction upon request.
The first step in safely performing botanical extraction is operating within the proper environment. While processing botanical extracts, it is not uncommon to be working with flammable solvents, toxic vapors, and vessels under pressure or vacuum. While there is always an inherent risk when dealing with these hazards, the level of risk can be greatly mitigated by handling these hazards within a controlled environment.
In order to maintain a controlled environment during the botanical extraction process, it is paramount to control the temperature, humidity, and airflow of the extraction and solvent storage areas. While some hazards will require operation in an appropriately rated hazardous location, it is important to maintain control of all areas of a botanical extraction lab to ensure the level of hazard is reduced as much as possible. For this reason, all botanical extraction and post-processing areas should be kept at a controlled temperature and humidity with appropriate exhaust ventilation.

16" Explosion-proof Ventilation Fan By Allegro Industries
While the temperature and humidity of the botanical extraction lab should be kept at a constant temperature between 60-73F/15.5C-23C with a relatively low humidity of around 20%, the level of ventilation within the botanical extraction lab will vary depending on the hazards being handled. While exhaust ventilation in a botanical extraction lab should always provide a high air exchange rate, it is best to regulate exhaust ventilation of a botanical extraction lab based on the level of flammable vapor within the surrounding area by utilizing a lower explosive limit detector.

Lower Explosive Limit Gas Detector By Honeywell
A lower explosive limit detector measures the level of gas or vapor within a given area and should be mounted 6" above the floor and set up to provide an auditory and visual alert when high concentrations of flammable vapors exist. A lower explosive limit detector should be used to trigger an increased rate of exhaust ventilation to keep vapors well below their lower explosive limit. When extracting with flammable solvents, it is best to keep the total percentage of solvent vapors below 25% of the solvents lower explosive limit of the solvent. The lower explosive limit of the solvents outlined in this course are as follows: Butane 1.86%, propane 2.1%, ethanol 3.3%, heptane 1.1%.
Portable Butane Leak Detector By BVV
Explosion mitigation is extremely important when performing solvent-based extraction. While the majority of the risk can be mitigated by performing extraction within an appropriately rated extraction booth or working with solvents under an appropriately rated fume hood, great care also needs to be taken in the storage of these solvents in an appropriately rated fire safe storage cabinet or within an appropriately rated hazardous location based on the solvents safety data sheet.
Hazardous locations are areas where the possibility of fire or explosion hazards may exist under normal or abnormal conditions because of the presence of flammable, combustible, or ignitable gases, vapors, liquids, dust, or fibers/flyings. These areas are classified solely to ensure proper specification and safe electrical/electronic equipment installation.

Hazardous-Location Enclosed Motor Switch By BVV
Hazardous locations fall under three classifications with two separate divisions. Class 1 locations are those in which flammable vapors may be present, Class 2 locations are those in which combustible dust may be found, and class 3 locations are those in which easily ignitable fibers or flyings may be present. Most solvent-based botanical extract processing techniques fall under the first classification due to the possibility of flammable vapors being present.
Depending on the specific botanical extract processing technique, it may be classified as division 1 or division 2; these divisions define the likelihood of hazardous materials in a flammable concentration. In a division 1 environment, ignitable concentrations of hazards exist under normal operating conditions or where a hazard is caused by frequent maintenance, repair work, or equipment failure. Division 2 environments are defined as environments where ignitable concentrations of hazards are handled, processed, or used, while normally in closed containers or closed systems from which they can only escape through accidental rupture or breakdown of such containers or systems.

C1D1 Extraction Booth By Advanced Extraction Labs
Hazardous locations require all electrical/electronics equipment to be designed, tested, and labeled as acceptable for use in the areas where it is installed. Beyond utilizing appropriately rated electrical/electronic components, most extraction areas will come equipped with an explosion-proof exhaust fan providing a high air exchange rate, along with a lower explosive limit gas detector to alert high levels of gas and trigger an increased rate of exhaust ventilation.
While the use of appropriately rated electrical components within an extraction area drastically lowers the potential of a flammable substance being ignited, one of the more critical functions of an extraction area is to reduce the risk of an explosion by regulating the airflow of the enclosed space to keep the levels of gases below the lower explosive limit of the used gas.
An appropriately rated extraction booth is a must-have to safely and compliantly perform solvent-based extraction. It is also required by most local municipalities and should be worked into the budget of every solvent-based extraction lab build. Depending on a specific municipality, fire inspectors may require said explosion-proof booths to have a fireproof burn rating typically required when dealing with larger volumes of solvent within the enclosed area. For this reason, it is always important to meet with the local fire inspector to determine the exact requirements that must be met to perform botanical extraction and store solvents compliantly.
Signage is an important factor in compliance, The NFPA requires a hazard rating diamond sign and no smoking signs shall be displayed in front of the extraction room door. While safety data sheets are an excellent standardized way to pass along this information. NFPA hazard diamonds provide are a quick reference for emergency responders so that they know what hazards they can expect when they arrive at the scene and can prepare appropriately.
The National Fire Association has developed a color-coded number system called NFPA 704. This system uses a color-coded diamond with four quadrants in which numbers are used in the upper three quadrants to signal the degree of health hazard (blue), flammability hazard (red), and reactivity hazard (yellow). The bottom quadrant is used to indicate special hazards. The NFPA system is good for alerting personally of the degree of hazard of the chemical and helpful in drawing attention to storage needs and the necessary emergency equipment needed.
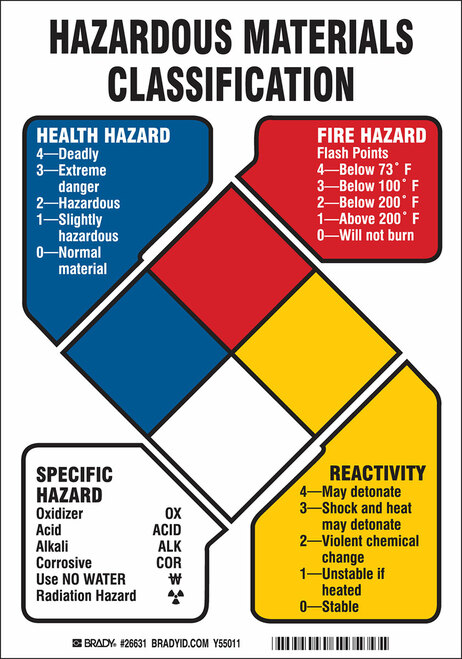
While the local fire marshall will determine the exact specifications a botanical extraction lab needs to meet, hydrocarbon extraction will typically require operation within an explosion-proof enclosure meeting C1D1 specifications, and ethanol extraction will typically require operation within an explosion-proof enclosure meeting C1D2 specifications.
Keep in mind, all non-appropriately rated electrical equipment should be kept outside of hazardous locations. All equipment that can be grounded should be, and when transferring flammable solvents from metal containers the containers should be bonded to limit static electricity. Metal containers can be easily bonded by securely attaching a metal bonding strap to both containers to prevent the build-up of static electricity.
All metal objects must be grounded to NEC code. Metal objects that are not grounded can create a dangerous spark that could cause a fire or explosion in the presence of flammable solvents. When a metal object is grounded, the excess charge is balanced by transferring electrical energy between the ground and the charged object the result is a safe return path for the charge between an object to the earth. When transferring flammable solvent from one container to another it is always best to bond the two containers together to limit the transfer of static electricity.
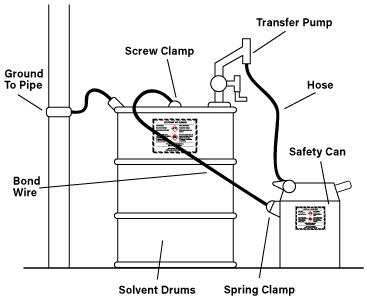
Bonding is the act of joining two or more electrical conductors together, Bonding must occur to the areas of equipment that are not intended to carry any current during normal operation. Bonding itself does not protect anything. However, if one of those pieces of equipment is grounded there can be no electrical energy build-up.
Grounding is connecting parts that carry current under normal conditions to the earth. Normally done for the protection of power system equipment and to provide an effective return path from the machine to the power source
Additionally, equipment should always be maintained by replacing gaskets and high-pressure clamp brass nuts regularly and always pressure testing pressure vessels. Before each use, always inspect nuts and bolts for wear and tear. Check the threads to ensure they have crisp edges. Inspect nuts for wear on the outside and that no cracking has occurred. THIS IS ESPECIALLY IMPORTANT ON CLAMPS THAT ARE REMOVED FREQUENTLY. If any wear and tear on the nuts occur, replace them immediately. Nuts will wear faster than bolts. It is recommended to change nuts every 2-4 weeks depending on the frequency of use.
Equipment is only as strong as its weakest link. When a gasket, nut, bolt, or clamp shows wear and tear it should be replaced immediately. It is also best practice to replace gaskets, nuts, bolts, and clamps regularly. Even valve seals and connections can deteriorate over time it is important to visually inspect all components prior to operation to ensure that pressure can be maintained during extraction. Change bolts every three months if being used daily. Evenly tighten on both sides not one at a time.

While it is of the utmost importance to maintain a controlled environment regarding temperature, humidity, and airflow of the extraction and solvent storage areas, keep in mind that botanical extracts are typically meant for consumption, and the appropriate steps should be taken to limit contamination from bacteria, and debris by maintaining a clean and sterile environment. To maintain a clean and sterile environment, all areas where botanical extracts are made should be cleaned and sanitized daily to limit any sources of contamination.
Maintaining a clean and organized lab should be a daily practice to ensure the manufactured products are safe from contamination. Lab design should be implemented to promote a streamlined process, increase productivity, and reduce the risk of potential hazards. All unnecessary items, cardboard, and powders should be kept outside the botanical extraction lab, and daily cleaning in the form of sweeping, mopping, and sterilization are great practices to maintain a sufficiently sterile operation ensuring the products made are free from contamination.
When cleaning, it is always best to start at the highest and farthest point from the door, and then work from left to right, and back to front to avoid cross-contamination. 200 proof ethanol or D-Limonene are a great cleaning agent for sterilizing metal utensils, glassware, and stainless steel apparatuses. Ethanol also works great to clean up any nonpolar oil that may have been spilled. For caked-on compounds that do not wash away with ethanol, hot water can be utilized to remove water-soluble organic compounds from boiling flasks and other glassware. Generally, a bleach solution can be utilized to clean workspaces and floors at the end of each day.Food & Lab grade 200 Proof Ethanol By BVV
While the cleanliness of the botanical extraction area dramatically reduces the potential for contamination of a botanical extract, it is also paramount to utilize the proper personal protective equipment to limit contamination of the botanical extract and ensure the safety of the operator. The proper personal protective equipment should always be worn at all times when performing botanical extraction to protect those performing botanical extraction.
The use of personal protective equipment or PPE is paramount in protecting the health and safety of both the extractor and consumer. Before carrying out processes and tasks, proper protective equipment must always be worn upon entering the botanical extraction lab. The following protective equipment is ALWAYS recommended for personal safety as well as the customer’s safety.
Hairnets prevent contamination of the product. Safety goggles or face shields prevent anything from entering the eyes. Static-resistant lab coats to prevent burns, static electricity, or contamination. Nitrile gloves or temperature-resistant gloves are needed to prevent contamination and burns, and a face mask or respirator is needed to prevent inhalation of toxic gas, vapor, or airborne contaminants. Additionally, OSHA requires training on proper use when utilizing respirators and even eye protection
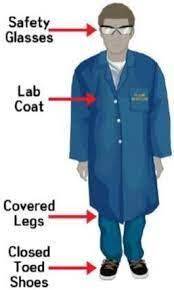
Beyond maintaining environmental control over the botanical extraction lab and wearing the proper personal protective equipment, maintaining process control is just as important to ensure the process is carried out consistently and safely limiting the level of hazard and potential for contamination of the botanical extract being created. Defining standard operating procedures for every process carried out in the lab is paramount in producing safe and consistent results while reducing potential hazards or sources of contamination.
While the standard operating procedures covered in the course are great starting points for performing botanical extraction, standard operating procedures should be developed for every process performed within the facility, from sanitizing equipment to taking out the trash. A procedure should be developed and implemented strictly for every process within the botanical extraction lab. Each employee should be trained how to perform each process to a T, and these procedures should be kept readily available within the lab for employees to review in order to perform the botanical extraction process safely and efficiently. While this section serves as an in-depth introduction to botanical extraction safety, we highly recommend an in-depth study of OSHA guidelines, Chapter 38 of the National Fire Protection Agency Fire Code. and NFPA 36 Standards for Sovent Extraction plants.


From foot scrubs to supplements, botanical extracts can be utilized across many industries providing increased effectiveness to a product in many use cases. Manufacturing products with botanical extracts inherently presents unique challenges that need to be appropriately addressed to manufacture products with consistency. This section will define some of the more important principles of manufacturing a product to maintain a level of repeatability and traceability based on Current Good Manufacturing Practices.
Manufacturing is turning raw materials into a component or finished product. Currently, in the US, The Food and Drug Administration regulates the standard for manufacturing drugs and supplements under the authority of the Federal Food, Drug, and Cosmetic Act. These regulations known as Current Good Manufacturing Practices or cGMP require that manufacturers, processors, and packagers of drugs & supplements (including botanical extracts) take proactive action to ensure that their products are safe, pure, and effective.
Good manufacturing practices start with the overall design of the building products will be manufactured in. A building used in the manufacturing, processing, packing, or holding of a drug product should be of suitable size, construction, and location to facilitate cleaning, maintenance, and proper operations. Any such building is required to have adequate space for the orderly placement of equipment and materials to prevent mix-ups between different components, drug product containers, closures, labeling, in-process material, or drug products to prevent contamination.
The building’s flow components, drug product containers, closures, labeling, in-process materials, and drug products should be designed to prevent contamination, and operations shall be performed within specifically defined areas of adequate size. There should be separate or defined areas or other control systems for the firm's operations as necessary to prevent contamination or mix-ups during manufacturing. On top of these more general building requirements, Good Manufacturing Practices have specific facility requirements covering lighting, ventilation, air filtration, air heating & cooling, plumbing, sewage, wash & toilet facilities, sanitation, maintenance, and equipment requirements.

Once these Building, Facility, & equipment requirements are met, the general focus of Good Manufacturing Practices is maintaining control and traceability of the manufacturing process. To maintain control and traceability over the manufacturing process, the implementation of Standard Operating Procedures (SOPs), batch recording, and internal tracking numbers are paramount.
SOP's are documents that outline every step in the manufacturing process. SOPs control the manufacturing process by ensuring that each manufacturing batch is manufactured in the same way providing consistency between batches. SOPs should be clearly defined for every step in the manufacturing process, starting with the initial receipt of biomass material to be extracted, all the way through the final distribution of the finished product, to ensure consistency.
Depending on the specific process, SOPs can vary in format & structure. The following are some of the key elements of an effective SOP. These key elements should be addressed in great detail and should include all the necessary information to complete the process from start to finish.
1. Purpose: a general overview of the process with a clearly defined desired outcome (EX. The purpose of this procedure is to provide detailed instructions for hydrocarbon extraction of botanical plant matter)
2. Scope: Defines to whom or what the particular set of procedures applies (EX. this procedure applies to all lab technicians tasked with hydrocarbon extraction).
3. Definitions: Clearly define any terms or acronyms used within the SOP (EX. Butane: Butane is a flammable nonpolar hydrocarbon solvent with a boiling point of -1C/30.2F
4. Safety & Hazard Identification: Define any potential hazards and safety protocols that are paramount to safely perform the process (EX. Butane is a highly flammable solvent, Butane extraction should be performed in an explosion-proof enclosure meeting C1D1 specifications.)
5. Preparation: A detailed step-by-step preparation procedure necessary to carry out the standard operating procedure. (EX. Before extraction, assemble and pressure test the extraction system with nitrogen to ensure no leaks.)
6. Procedure: A detailed step-by-step procedure clearly defining the exact process the operator should follow to obtain the desired outcome (EX. Step 4 -Open the material column's solvent injection valve located atop the material column injecting 5lbs of butane for every 1lb of biomass material loaded within the column.)
7. Revision History: A place to record the changes made to said procedure along with a justification or the reason why the procedure was edited

Along with Clearly defined and optimized SOPs, the entirety of the manufacturing process should be painstakingly recorded within a log of batch records. Batch records should be designed to log all the key data about the manufacturing process. Keeping intensive batch records and logging each specific detail of the manufacturing process associated with every batch made helps improve consistency between batches and reduce manufacturing errors.
Batch logs can ensure that manufacturing standards are maintained and provide in-depth information on each batch in the event of a defective product or recall. The key to successful batch recording is recording the proper metrics. While the relevant metrics and data to be recorded in batch records will vary from product to product, below are some ideas of relevant data worth capturing.
Where was each ingredient sourced from?
When was each ingredient sourced?
What is the internal batch number for this ingredient?
What was the quality or characteristics of the ingredient?
What equipment was used to manufacture the product?
What steps were performed?
How much of each component was added?
How long was the duration of the process?
When was the process performed?
Who performed the process?
A description of the final product
The exact number of units produced
Documentation of quality testing

In consideration of maintaining comprehensive batch records during the manufacturing process, each input and byproduct of the manufacturing process should be assigned an internal tracking number so each batch of the finished product can be associated with the specific ingredients that were used to make it so one can provide batch traceability in the event of a defective product or recall.
When batch recording is done correctly, one should be able to look back at any manufactured product and know the exact time it was made, who made it, what ingredients were used, where the ingredients were sourced, what batches were made with those ingredients, and where the remaining products from that batch were sold in the case of a recall. This level of recording allows for complete chain traceability of a manufactured good, from sourcing the raw ingredients to the sale of the final product.
By implementing these manufacturing guidelines, strong quality management systems, and analytical testing standards, consistent and traceable products can be produced. While this section serves as an introduction to Good Manufacturing Practices, it still only scratches the surface. For more information on Good Manufacturing Practices, refer to Title 21 of the Code of Federal Regulations Part 111.
