Description
BVV™ High Purity Lab Grade Methanol
HAZMAT ITEMS ARE NON-REFUNDABLE. ALL SALES ARE FINAL
(Note: Container style and color may vary)
- Spigots/Faucets are only compatible with 5 Gallon Plastic Jugs and must be purchased separately
BVV™ High Purity Lab Grade Methanol is used to remove and extract terpenes, tannins, alkaloids, glycosides, lignans, and terpinoids from plants. It is used to extract bio-active, phenolic and polar compounds from medicinal plants. Methanol is a high purity solvent used to manufacture botanical solutions and is easily evaporated. Methanol is used in research and testing laboratories for phytochemical study, phytochemical analysis and chemical synthesis. Methanol is used to prepare methanol/water/acetic acid mixtures.
| Chemical Formula: |
CH3OH |
| Molecular Weight: |
32.042 g·mol−1
|
| CAS Registry Number: |
67-56-1 |
| Appearance |
Colorless Liquid |
| Odor: |
Faint and similar to ethanol |
| Density |
0.792 g/cm3
|
| Boiling Point: |
64.7 °C /148.5 °F |
| Solubility in water: |
miscible |
| GHS Pictograms: |
  
|
| GHS Signal Word: |
Danger |
| GHS Hazard Statements: |
H225, H301, H302, H305, H311, H331, H370 |
| GHS Precautionary Statements |
P210, P233, P235, P240, P241, P242, P243, P260, P264, P270, P271, P280, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P307+P311, P310, P311, P312, P337+P313, P361, P363, P370+P378, P403+P233, P405, P501 |
| UN Identification Number: |
1230 |
| Proper Shipping Name: |
Methanol |
| Transport Hazard Class: |
3 |
| Packing Group: |
II |
| DOT Placard: |
 |
What Is Methanol?
Methanol, also known as methyl alcohol or wood alcohol, is a type of alcohol with the chemical formula CH3OH. It is the simplest alcohol, consisting of a methyl group (CH3) linked to a hydroxyl group (OH). Methanol is a colorless, flammable liquid with a slightly sweet odor. It is commonly used as an industrial solvent, antifreeze, fuel, and as a feedstock in the production of chemicals, plastics, and synthetic materials.
Methanol can be synthesized from various sources, including natural gas, carbon monoxide, and biomass. It is considered a hazardous substance due to its toxicity when ingested, inhaled, or absorbed through the skin. Methanol is also used as an industrial and laboratory solvent and as a fuel in some types of racing cars and model engines.
One important thing to note is that methanol is highly toxic when consumed, and even small amounts can be lethal. It should never be ingested, and safety precautions should be taken when handling this substance.
What Is Methanol Used For?
Methanol is used for a wide range of industrial, commercial, and laboratory applications. Some of its common uses include:
-
Fuel: Methanol is used as an alternative fuel in some types of vehicles, particularly racing cars and model engines. It is also used in the production of biodiesel and as a fuel additive.
-
Solvent: Methanol is a versatile solvent that can dissolve a variety of substances, making it useful in industries such as paint, varnish, and ink production. It is also used as a cleaning agent in laboratories and industrial settings.
-
Antifreeze: Methanol is an essential component of some antifreeze formulations, where it helps prevent the freezing of engine coolant in cold temperatures.
-
Chemical Intermediate: Methanol serves as a key building block in the production of various chemicals and materials, including formaldehyde, acetic acid, methyl methacrylate (used in plastics and coatings), and more.
-
Fuel Cell Feedstock: Methanol can be used as a feedstock for the production of hydrogen, which can be used in fuel cells for electricity generation and other applications.
-
Preservative: In the pharmaceutical and personal care industries, methanol is used as a preservative in some products.
-
Denaturant: Methanol is added to industrial ethanol to make it unfit for consumption (denatured). This ensures that the ethanol cannot be used for drinking purposes and is used for industrial applications.
-
Laboratory Reagent: Methanol is commonly used in laboratories as a reagent and solvent for various chemical experiments and analyses.
-
Extraction: It is used in some extraction processes, such as the extraction of essential oils from plants.
-
Energy Storage: Methanol is being explored as a potential energy carrier for energy storage and transportation, particularly in the form of methanol fuel cells.
It's important to note that methanol is toxic to humans and should be handled with care. Ingesting or inhaling methanol vapors can be extremely dangerous and potentially fatal. Proper safety precautions and handling procedures are necessary when working with methanol.
What Is The Structure of Methanol?
Methanol, also known as methyl alcohol, has a simple chemical structure. Its molecular formula is CH3OH, which represents one carbon (C) atom, four hydrogen (H) atoms, and one oxygen (O) atom bonded together. Here's the structural formula of methanol:
In this structure:
- The central carbon atom (C) is bonded to three hydrogen atoms (H) and one oxygen atom (O).
- The oxygen atom is bonded to the carbon atom through a single covalent bond (C-O).
- The remaining three hydrogen atoms are bonded to the carbon atom, completing its four covalent bonds.
Methanol is a polar molecule due to the electronegativity difference between oxygen and hydrogen atoms, which results in a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom. This polarity gives methanol its unique chemical properties and makes it a versatile solvent in various applications.
What are the Hazards of Methanol?
Methanol, while commonly used in various industrial and laboratory applications, poses several hazards, primarily due to its toxic and flammable nature. Here are some of the hazards associated with methanol:
-
Toxicity: Methanol is highly toxic to humans when ingested, inhaled, or absorbed through the skin. The toxic effects are primarily due to its metabolites, formaldehyde, and formic acid. Methanol poisoning can lead to symptoms such as headache, dizziness, nausea, vomiting, abdominal pain, and in severe cases, it can cause blindness, organ failure, and death.
-
Flammability: Methanol is flammable and can form explosive mixtures in the air when its vapor concentration is within certain limits. It has a relatively low flashpoint, making it susceptible to ignition by heat, sparks, or open flames. Proper storage and handling precautions are essential to prevent fire hazards.
-
Irritant: Methanol can be irritating to the eyes, skin, and respiratory tract. Contact with methanol vapor or liquid can lead to skin irritation, redness, and chemical burns. Inhaling methanol vapor can irritate the respiratory system and cause coughing and throat irritation.
-
Environmental Impact: Methanol is harmful to the environment. Spills or releases of methanol can contaminate soil and water, posing a risk to aquatic life and ecosystems. It is important to handle and dispose of methanol responsibly to minimize its environmental impact.
-
Incompatibility: Methanol should not be stored or transported in containers or systems that have previously held incompatible materials, as it can react with some substances and form hazardous compounds.
-
Cumulative Exposure: Prolonged or repeated exposure to methanol vapor or mist over time can result in cumulative health effects, particularly on the central nervous system and the optic nerve.
-
Ingestion Risk: Methanol has a sweet taste and is sometimes mistaken for ethanol (the alcohol found in alcoholic beverages). Accidental ingestion of methanol can occur, especially if it is stored in containers that are not clearly labeled.
To safely handle methanol and mitigate these hazards, it is crucial to follow strict safety protocols, use appropriate personal protective equipment (PPE), store methanol in well-ventilated areas away from open flames, and provide proper training to personnel working with methanol. Emergency response plans and first-aid measures for methanol exposure should also be in place in case of accidents or spills.
How Do I Use Methanol Safely?
Using methanol safely is essential due to its toxic and flammable nature. Whether you're working with methanol in a laboratory, industrial setting, or other applications, here are some guidelines for safe handling:
-
Personal Protective Equipment (PPE):
- Always wear appropriate PPE, including safety goggles, chemical-resistant gloves, a lab coat or chemical-resistant apron, and closed-toe shoes.
- Consider additional protective gear, such as a face shield, when handling large quantities or performing high-risk operations.
-
Storage:
- Store methanol in well-ventilated areas away from heat sources, open flames, and direct sunlight.
- Use containers made of materials compatible with methanol, such as glass or approved plastic containers. Label containers clearly with the contents.
- Ensure proper labeling and hazard signage in storage areas.
-
Handling:
- Handle methanol in a fume hood or well-ventilated workspace to minimize inhalation exposure.
- Do not use methanol near open flames, sparks, or heat sources.
- Avoid skin contact; wear gloves and lab coats to prevent skin exposure.
- Use a face shield or safety goggles to protect your eyes.
-
Spill Response:
- Have spill kits and absorbent materials (e.g., spill pads, spill pillows) available for immediate use.
- In the event of a spill, ventilate the area, and contain the spill to prevent it from spreading.
- Wear appropriate PPE when cleaning up spills.
- Follow your workplace's spill response procedures and dispose of contaminated materials properly.
-
Storage and Dispensing:
- Use safety containers designed for methanol when dispensing or transferring the liquid.
- Never use glassware or containers that have been used for other chemicals without thorough cleaning.
- Ensure proper grounding and bonding when transferring methanol to prevent static electricity buildup.
-
Inhalation Exposure:
- Minimize inhalation exposure by working in a well-ventilated area or under a fume hood.
- If you suspect inhalation exposure, move to an area with fresh air and seek medical attention if symptoms persist.
-
Ingestion Prevention:
- Do not eat, drink, or smoke in areas where methanol is handled.
- Avoid using containers or equipment that may be contaminated with methanol for food or beverage storage.
-
Fire Safety:
- Methanol is flammable; keep it away from open flames, sparks, and heat sources.
- Use explosion-proof electrical equipment in areas where methanol vapors may be present.
-
Emergency Response:
- Familiarize yourself with the location of safety showers, eyewash stations, fire extinguishers, and emergency exits.
- Know the location of first-aid supplies and procedures for methanol exposure.
-
Training and Education:
- Ensure that personnel handling methanol are properly trained in its safe handling, storage, and emergency response procedures.
-
Waste Disposal:
- Dispose of methanol waste in accordance with local, state, and federal regulations for hazardous waste disposal.
Always follow your workplace's safety protocols and consult with safety officers or supervisors for specific safety procedures related to methanol handling in your environment. Regular safety training and awareness are essential to prevent accidents and protect your health when working with methanol.
$27.00
You will earn 27 points for this purchase.
✔ Made in the USA
- Want it cheaper? Call us for a wholesale or processor discount. 331-281-0154
Description
BVV™ High Purity Lab Grade Methanol
HAZMAT ITEMS ARE NON-REFUNDABLE. ALL SALES ARE FINAL
(Note: Container style and color may vary)
- Spigots/Faucets are only compatible with 5 Gallon Plastic Jugs and must be purchased separately
BVV™ High Purity Lab Grade Methanol is used to remove and extract terpenes, tannins, alkaloids, glycosides, lignans, and terpinoids from plants. It is used to extract bio-active, phenolic and polar compounds from medicinal plants. Methanol is a high purity solvent used to manufacture botanical solutions and is easily evaporated. Methanol is used in research and testing laboratories for phytochemical study, phytochemical analysis and chemical synthesis. Methanol is used to prepare methanol/water/acetic acid mixtures.
| Chemical Formula: |
CH3OH |
| Molecular Weight: |
32.042 g·mol−1
|
| CAS Registry Number: |
67-56-1 |
| Appearance |
Colorless Liquid |
| Odor: |
Faint and similar to ethanol |
| Density |
0.792 g/cm3
|
| Boiling Point: |
64.7 °C /148.5 °F |
| Solubility in water: |
miscible |
| GHS Pictograms: |
  
|
| GHS Signal Word: |
Danger |
| GHS Hazard Statements: |
H225, H301, H302, H305, H311, H331, H370 |
| GHS Precautionary Statements |
P210, P233, P235, P240, P241, P242, P243, P260, P264, P270, P271, P280, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P307+P311, P310, P311, P312, P337+P313, P361, P363, P370+P378, P403+P233, P405, P501 |
| UN Identification Number: |
1230 |
| Proper Shipping Name: |
Methanol |
| Transport Hazard Class: |
3 |
| Packing Group: |
II |
| DOT Placard: |
 |
What Is Methanol?
Methanol, also known as methyl alcohol or wood alcohol, is a type of alcohol with the chemical formula CH3OH. It is the simplest alcohol, consisting of a methyl group (CH3) linked to a hydroxyl group (OH). Methanol is a colorless, flammable liquid with a slightly sweet odor. It is commonly used as an industrial solvent, antifreeze, fuel, and as a feedstock in the production of chemicals, plastics, and synthetic materials.
Methanol can be synthesized from various sources, including natural gas, carbon monoxide, and biomass. It is considered a hazardous substance due to its toxicity when ingested, inhaled, or absorbed through the skin. Methanol is also used as an industrial and laboratory solvent and as a fuel in some types of racing cars and model engines.
One important thing to note is that methanol is highly toxic when consumed, and even small amounts can be lethal. It should never be ingested, and safety precautions should be taken when handling this substance.
What Is Methanol Used For?
Methanol is used for a wide range of industrial, commercial, and laboratory applications. Some of its common uses include:
-
Fuel: Methanol is used as an alternative fuel in some types of vehicles, particularly racing cars and model engines. It is also used in the production of biodiesel and as a fuel additive.
-
Solvent: Methanol is a versatile solvent that can dissolve a variety of substances, making it useful in industries such as paint, varnish, and ink production. It is also used as a cleaning agent in laboratories and industrial settings.
-
Antifreeze: Methanol is an essential component of some antifreeze formulations, where it helps prevent the freezing of engine coolant in cold temperatures.
-
Chemical Intermediate: Methanol serves as a key building block in the production of various chemicals and materials, including formaldehyde, acetic acid, methyl methacrylate (used in plastics and coatings), and more.
-
Fuel Cell Feedstock: Methanol can be used as a feedstock for the production of hydrogen, which can be used in fuel cells for electricity generation and other applications.
-
Preservative: In the pharmaceutical and personal care industries, methanol is used as a preservative in some products.
-
Denaturant: Methanol is added to industrial ethanol to make it unfit for consumption (denatured). This ensures that the ethanol cannot be used for drinking purposes and is used for industrial applications.
-
Laboratory Reagent: Methanol is commonly used in laboratories as a reagent and solvent for various chemical experiments and analyses.
-
Extraction: It is used in some extraction processes, such as the extraction of essential oils from plants.
-
Energy Storage: Methanol is being explored as a potential energy carrier for energy storage and transportation, particularly in the form of methanol fuel cells.
It's important to note that methanol is toxic to humans and should be handled with care. Ingesting or inhaling methanol vapors can be extremely dangerous and potentially fatal. Proper safety precautions and handling procedures are necessary when working with methanol.
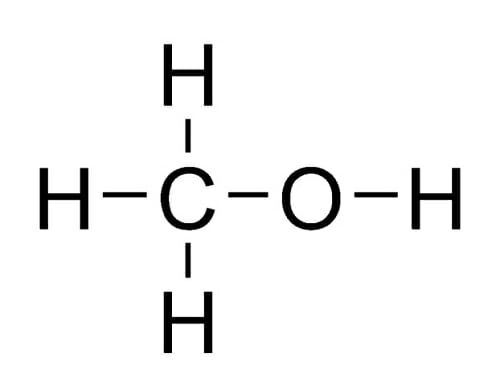
What Is The Structure of Methanol?
Methanol, also known as methyl alcohol, has a simple chemical structure. Its molecular formula is CH3OH, which represents one carbon (C) atom, four hydrogen (H) atoms, and one oxygen (O) atom bonded together. Here's the structural formula of methanol:
In this structure:
- The central carbon atom (C) is bonded to three hydrogen atoms (H) and one oxygen atom (O).
- The oxygen atom is bonded to the carbon atom through a single covalent bond (C-O).
- The remaining three hydrogen atoms are bonded to the carbon atom, completing its four covalent bonds.
Methanol is a polar molecule due to the electronegativity difference between oxygen and hydrogen atoms, which results in a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom. This polarity gives methanol its unique chemical properties and makes it a versatile solvent in various applications.
What are the Hazards of Methanol?
Methanol, while commonly used in various industrial and laboratory applications, poses several hazards, primarily due to its toxic and flammable nature. Here are some of the hazards associated with methanol:
-
Toxicity: Methanol is highly toxic to humans when ingested, inhaled, or absorbed through the skin. The toxic effects are primarily due to its metabolites, formaldehyde, and formic acid. Methanol poisoning can lead to symptoms such as headache, dizziness, nausea, vomiting, abdominal pain, and in severe cases, it can cause blindness, organ failure, and death.
-
Flammability: Methanol is flammable and can form explosive mixtures in the air when its vapor concentration is within certain limits. It has a relatively low flashpoint, making it susceptible to ignition by heat, sparks, or open flames. Proper storage and handling precautions are essential to prevent fire hazards.
-
Irritant: Methanol can be irritating to the eyes, skin, and respiratory tract. Contact with methanol vapor or liquid can lead to skin irritation, redness, and chemical burns. Inhaling methanol vapor can irritate the respiratory system and cause coughing and throat irritation.
-
Environmental Impact: Methanol is harmful to the environment. Spills or releases of methanol can contaminate soil and water, posing a risk to aquatic life and ecosystems. It is important to handle and dispose of methanol responsibly to minimize its environmental impact.
-
Incompatibility: Methanol should not be stored or transported in containers or systems that have previously held incompatible materials, as it can react with some substances and form hazardous compounds.
-
Cumulative Exposure: Prolonged or repeated exposure to methanol vapor or mist over time can result in cumulative health effects, particularly on the central nervous system and the optic nerve.
-
Ingestion Risk: Methanol has a sweet taste and is sometimes mistaken for ethanol (the alcohol found in alcoholic beverages). Accidental ingestion of methanol can occur, especially if it is stored in containers that are not clearly labeled.
To safely handle methanol and mitigate these hazards, it is crucial to follow strict safety protocols, use appropriate personal protective equipment (PPE), store methanol in well-ventilated areas away from open flames, and provide proper training to personnel working with methanol. Emergency response plans and first-aid measures for methanol exposure should also be in place in case of accidents or spills.
How Do I Use Methanol Safely?
Using methanol safely is essential due to its toxic and flammable nature. Whether you're working with methanol in a laboratory, industrial setting, or other applications, here are some guidelines for safe handling:
-
Personal Protective Equipment (PPE):
- Always wear appropriate PPE, including safety goggles, chemical-resistant gloves, a lab coat or chemical-resistant apron, and closed-toe shoes.
- Consider additional protective gear, such as a face shield, when handling large quantities or performing high-risk operations.
-
Storage:
- Store methanol in well-ventilated areas away from heat sources, open flames, and direct sunlight.
- Use containers made of materials compatible with methanol, such as glass or approved plastic containers. Label containers clearly with the contents.
- Ensure proper labeling and hazard signage in storage areas.
-
Handling:
- Handle methanol in a fume hood or well-ventilated workspace to minimize inhalation exposure.
- Do not use methanol near open flames, sparks, or heat sources.
- Avoid skin contact; wear gloves and lab coats to prevent skin exposure.
- Use a face shield or safety goggles to protect your eyes.
-
Spill Response:
- Have spill kits and absorbent materials (e.g., spill pads, spill pillows) available for immediate use.
- In the event of a spill, ventilate the area, and contain the spill to prevent it from spreading.
- Wear appropriate PPE when cleaning up spills.
- Follow your workplace's spill response procedures and dispose of contaminated materials properly.
-
Storage and Dispensing:
- Use safety containers designed for methanol when dispensing or transferring the liquid.
- Never use glassware or containers that have been used for other chemicals without thorough cleaning.
- Ensure proper grounding and bonding when transferring methanol to prevent static electricity buildup.
-
Inhalation Exposure:
- Minimize inhalation exposure by working in a well-ventilated area or under a fume hood.
- If you suspect inhalation exposure, move to an area with fresh air and seek medical attention if symptoms persist.
-
Ingestion Prevention:
- Do not eat, drink, or smoke in areas where methanol is handled.
- Avoid using containers or equipment that may be contaminated with methanol for food or beverage storage.
-
Fire Safety:
- Methanol is flammable; keep it away from open flames, sparks, and heat sources.
- Use explosion-proof electrical equipment in areas where methanol vapors may be present.
-
Emergency Response:
- Familiarize yourself with the location of safety showers, eyewash stations, fire extinguishers, and emergency exits.
- Know the location of first-aid supplies and procedures for methanol exposure.
-
Training and Education:
- Ensure that personnel handling methanol are properly trained in its safe handling, storage, and emergency response procedures.
-
Waste Disposal:
- Dispose of methanol waste in accordance with local, state, and federal regulations for hazardous waste disposal.
Always follow your workplace's safety protocols and consult with safety officers or supervisors for specific safety procedures related to methanol handling in your environment. Regular safety training and awareness are essential to prevent accidents and protect your health when working with methanol.