
Chapter 2: Solvent Based Extraction (Part 2: Ethanol Extraction)
, by Avery Benitez, 27 min reading time

, by Avery Benitez, 27 min reading time

ETHANOL EXTRACTION

45L Jacketed Stainless Steel centrifuge By BVV
Ethanol extraction is an excellent method of botanical extraction valued for its high throughput potential and reduced flammability compared to more volatile solvent-based extraction methods like hydrocarbon extraction. Ethanol is classified as alcohol made from starch-based plant materials through a complex fermentation and distillation process. Ethanol (EtOH), commonly known as ethyl alcohol or grain alcohol, is a colorless flammable liquid with an incredible number of uses beyond botanical extraction including fuel and a cleaning agent.
As an extraction solvent ethanol is unique due to its amphiphilic nature meaning it has both polar and nonpolar properties. Ethanol has a hydrotropic polar head (water-loving) that can bind to water-soluble compounds, and a nonpolar tail that can dissolve hydrophobic or oil-soluble compounds typically found in botanical biomass. As the plant matter is saturated with ethanol, it dissolves both polar and nonpolar compounds from the biomass material extracting them. Ethanol's amphiphilic nature can be ideal when seeking to create a more full-spectrum product, but it can be less than ideal for those looking to create a more concentrated extract.
Due to ethanols' polar and nonpolar nature, it has an increased propensity to co-extract undesirable plant waxes and chlorophyll during the extraction process. This typically results in a less potent and pure end product requiring more post-processing to reach the same purity level than that of a nonpolar solvent like butane or propane. While ethanol’s selectivity for undesirable plant waxes and chlorophyll can be altered by chilling the ethanol prior to extraction, reducing the solubility of undesirable plant waxes and color pigments, it also reduces the solubility of target compounds as well.

475L Jacketed Stainless Steel Centrifuge By BVV
Beyond its polarity, ethanol has a high saturation capacity and only requires a short retention time to dissolve target compounds lending itself to short runtimes and high throughput potential. Ethanol is particularly well suited for bulk processing, with available systems that can extract thousands of pounds of biomass within a single working shift. This makes ethanol a great option for those making botanical extracts at larger scales.
While ethanol extraction can be extremely well suited for some applications, it may not be the best suited for applications with low budgetary requirements or when targeting lower boiling point botanical compounds. These factors are typically the concern when it comes to the solvent recovery process. Ethanol extractions typically require large amounts of ethanol and large-scale solvent recovery systems to process efficiently. Unlike hydrocarbon extraction where the bulk of the solvent recovery process happens within the same extraction apparatus, ethanol solvent recovery is typically performed in a separate ancillary solvent recovery system like a rotary evaporator or falling film evaporator.
When it comes to ethanol solvent recovery, Ethanols boiling point of 78C/173F is considerably higher than that of light hydrocarbons. While ethanols boiling point still makes the solvent easily recovered from the botanical extract it may be conflicting with some lower boiling point or highly volatile target compounds. Although ethanols boiling point is quite a bit higher in comparison to hydrocarbon boiling points it is typically well below most botanical compounds making it a considerable candidate for most botanical extractions.
Aside from ancillary solvent recovery equipment ethanol extraction typically requires other ancillary post-processing procedures in the form of winterization/filtration and distillation in order to achieve a high purity extract. While this additional post-processing equipment drastically raises the startup cost of ethanol extraction it's well worth it when included in the ethanol processing flow resulting in a highly refined and potent botanical concentrate.
270 Gallon Tote of 200 Proof Food & Lab Grade Ethanol By BVV
Ethanol can come in a variety of different types, which can be utilized for botanical extraction commonly classified by their proof which designates their purity and their potability, meaning its suitability for drinking. Commonly ethanol is made non-potable by denaturing it with a substance that discourages people from consuming it recreationally. While denatured ethanol is unfit for consumption, it can be utilized effectively for botanical extraction resulting in a drastic reduction in extraction cost by circumventing the Federal Excise Tax on alcohol.
The most commonly used types of ethanol employed for botanical extraction include 200 proof ethanol (pure ethanol), potable 190 proof ethanol (containing 5% water), and 190 proof n-heptane denatured ethanol (containing 5% n-Heptane). These extraction-grade ethanols can be efficiently used to extract both polar and nonpolar botanical compounds. While 200 proof ethanol is great to ensure the extraction solvent is of the highest purity, both potable 190 proof ethanol and n-heptane denatured ethanol have their own unique benefits.
Potable 190 proof ethanol contains roughly 5% water by mass. This small amount of water makes the ethanol slightly more polar than 200 proof ethanol and forms an azeotrope between the ethanol and water, decreasing its boiling point ever so slightly. This increased polarity reduces affinity towards most target nonpolar botanical compounds and undesirable nonpolar plant waxes. While this increased polarity can benefit when utilized in conjunction with chilling the solvent prior to extraction to limit the pickup of unwanted plant waxes and lipids, this is somewhat counterintuitive when targeting nonpolar botanical compounds but nonetheless can be utilized to create higher purity ethanol extracted botanical concentrate. 190 proof ethanol is commonly the preferred type of ethanol when extracting cold for its excellent selectivity at chilled temperatures beyond -40C/-40F.
5 Gallon Ultra High Purity 710 Extraction Solvent By BVV
Conversely, 190 proof n-heptane denatured ethanol can be utilized, 190 proof n-heptane denatured ethanol similarly result in an azeotrope between the ethanol and n-heptane, but due to n-heptanes non-polarity, this increases its affinity to extract target nonpolar botanical compounds. Additionally, 190 proof n-heptane denatured ethanol allows for the Federal Excise Tax on alcohol to be circumvented, drastically reducing the cost of using ethanol as an extraction solvent.
55 Gallon Drum of CDA-12A Denatured Ethanol (n-Heptane Denatured) By BVV
Both potable and n-Heptane denatured 190 proof ethanol create an azeotrope between the ethanol and either the water or n-heptane. An azeotrope is defined as a mixture of two liquids that have a constant boiling point and composition through distillation. This azeotrope results in the ethanol and either water or n-heptane evaporating at the same temperature, making it extremely difficult to separate the two through distillation. While these azeotropic solvents are still easily recovered from a botanical extract at a relatively low boiling point around 78C/173F using a rotary evaporator or falling film evaporator, they can be complicated to reuse after absorbing additional water from biomass material.

4A Molecular Sieve Beads By mSORB
While all types of extraction grade ethanol can be easily distilled from a botanical extract to non-detectable levels, reuse proves challenging due to the pickup of additional water from the biomass material. Additional water absorbed by the solvent negatively affects its extraction efficiency over time and requires reproofing using 4A molecular sieve to remove the excess water. Reproofing is especially important with n-heptane denatured ethanol as reuse results in a ternary (3-part) azeotrope between the ethanol, n-heptane, and the additional water absorbed over time, resulting in dramatically reduced extraction efficiency.
Walk-In Fume Hood By Fisher American
While non-denatured forms of ethanol are Generally Regarded as Safe (GRAS) by the FDA and are typically consumed regularly within alcohol by many, it can be toxic to humans in high concentrations. When ethanol is used as an extraction solvent, this highly flammable liquid can be dangerous and requires careful storage and use. While it is highly unlikely that ethanol extraction will result in an explosion when performed properly, when utilizing this highly flammable liquid for extraction, there is always an inherent potential for ignition. For this reason, any and all potential sources of heat or flames should be kept far away from areas in which ethanol liquid or vapors are present.
Additionally, the temperature and airflow of storage and extraction areas should be carefully managed to reduce the level of flammable ethanol vapors as low as possible. Keeping the ethanol stored in a relatively cool area with good airflow reduces the release and level of flammable vapors in the atmosphere. By controlling the temperature and airflow of storage and extraction areas, the concentration of vapors can be reduced, which drastically lowers the potential of vapor ignition. To further mitigate the potential for ignition, ethanol should always be stored in an appropriate fireproof solvent cabinet, and extraction should be performed inside of a C1D2 extraction booth with all the proper safety and protective equipment.
Among the most important pieces of safety equipment for ethanol extraction is a C1D2 rated extraction booth which greatly reduces the potential hazard when working with ethanol as a solvent. A C1D2 hazardous location is an area where ignitable concentrations of vapor are handled, processed, or used, normally in closed containers or closed systems from which they can only escape through accidental rupture or breakdown of such containers or systems.
Mega Deluxe Series C1D1 Indoor Booth By Advanced Extraction Labs
Ethanol extraction typically falls under a Class 1 Division 2 hazardous location because ignitable concentrations of vapor are handled, processed, or used, while normally in closed containers or closed systems from which they can only escape through accidental rupture or breakdown of such containers or systems. Depending on the specific municipality, a fire inspector may also require said extraction booth to have a fireproof burn rating which is commonly needed when dealing with larger volumes of solvent within the enclosed area.
C1D2 Hazardous locations require all electrical/electronics equipment to be designed, tested, and labeled as being acceptable for use in C1D2 areas. Beyond utilizing appropriately rated electrical/electronic components, most C1D2 extraction booths will come equipped with an explosion-proof exhaust fan providing a high air exchange rate, along with a lower explosive limit gas detector to alert of high levels of gas and trigger an increased rate of exhaust ventilation.
While the use of appropriately rated electrical components within the extraction area drastically lowers the potential of a flammable substance being ignited, One of the more important functions of a C1D2 extraction area is to reduce the risk of an explosion by regulating the airflow of the enclosed space to keep the levels of gases below the lower explosive limit of the used gas.
Lower Explosive Limit Gas Detector By Honeywell
A C1D2 extraction booth helps mitigate the risk of an explosion through the use of an explosion-proof exhaust fan providing a high rate of air exchange, along with a lower explosive limit gas detector used to alert the user of high levels of gas and trigger an increased rate of exhaust ventilation. When extracting with ethanol it's best to keep the percentage solvent vapors within the enclosure well below 25% of their lower explosive limit, which is 3.3% for ethanol, through adequate ventilation.
When it comes to the storage of ethanol, OSHA requires that ethanol vapors be held below 25% of the 3.3% lower explosive limit through adequate ventilation. Commonly, the maximum amount of ethanol that can be stored in a flammable storage cabinet is 60 gallons, and the maximum storage permissible outside of a flammable cabinet or storage room is 25 gallons. While the storage requirements may be different in certain municipalities, typically, any volume of ethanol greater than 200 gallons is required to be stored in a C1D2 space with a 5-hour fire-rated wall. It is important to work with the local fire marshal to determine the exact requirements for ethanol use and storage within the facility and get proper approval from the municipality to store ethanol and perform ethanol extraction.
Keep in mind, when performing an ethanol extraction inside a C1D2 rated hazardous location, only appropriately rated explosion-proof electronics should be utilized, and great care should be taken to limit exposure to open flame or static electricity. A C1D2 Environment is a must-have to safely and competently perform ethanol extraction and should be worked into the budget of every new ethanol extraction lab build. While a C1D2 Extraction booth greatly reduces risk during ethanol extraction, to ensure personal safety while performing ethanol extraction, personal protective equipment in the form of safety goggles, nitrile gloves, antistatic clothing, and a respirator should be worn at all times when working with ethanol or where high concentrations of ethanol vapor exist.
Certified 160L Jacketed Stainless Steel Centrifuge with Explosion Proof Motor and Siemens Controller By BVV
When operating within a C1D2 environment, ethanol extraction is performed utilizing a grounded explosion-proof centrifuge by first bringing the solvent to the desired extraction temperature and injecting it into the ethanol centrifuge from a bonded container to completely submerge the biomass material. The biomass material is then agitated to increase the saturation of the biomass before draining the system and spinning the biomass at high rpm to further separate the remaining solution from the biomass material.
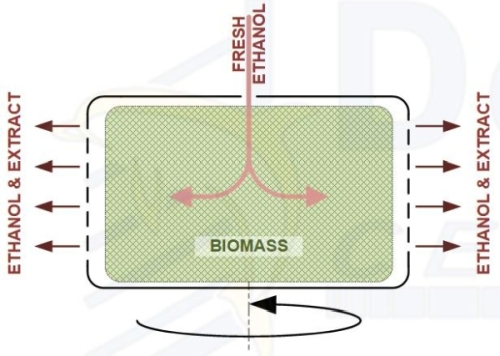
Ethanol extraction is performed simply by saturating the biomass material with ethanol solvent. As the liquid solvent saturates the biomass, it dissolves both polar and nonpolar compounds from the plant material. While this process is rather straightforward, there are several variables to the initial saturation that can be optimized to increase the overall yield and purity of the resulting extract. During the initial saturation, the most effective variables to adjust in order to optimize the efficiency of a centrifugal ethanol extraction are solvent temperature and retention time.
Solvent temperature is commonly altered either to increase the purity or overall yield of the ethanol extraction. The extractor may choose to either chill the solvent -30C to -40C (-22F to -40F) to limit the pickup of impurities or extract using a room temperature or "warm" solvent (15-30C/59-86F) to increase the overall yield of the extract. During extraction, the temperature of a solvent plays a big role in the solubility and saturation capacity of a solvent. Generally, both saturation capacity and solubility increase or decrease along with the temperature of the solvent.
When a solvent is chilled prior to extraction, its overall solubility is reduced, which helps limit the pickup of undesirable impurities like plant waxes and chlorophyll. While this altered selectivity does come at a price of reduced efficiency in extracting the target compounds, it greatly increases the purity of the resulting extract.
12"x24" Baffled Tri-Clamp Condenser/Heat Exchanger by BVV
When ethanol is chilled to temperatures below -40C/-40F, the water-resistant nonpolar plant waxes have significantly reduced solubility within the polar ethanol. While plant waxes are particularly more insoluble in the more polar potable 190 proof ethanol, plant wax solubility in 200 proof or n-heptane denatured ethanol is dramatically reduced at this temperature. This lower solubility of plant waxes within chilled ethanol is the same concept utilized to precipitate plant waxes from an ethanol solution during the process of winterization, which will be covered in the next chapter.
As ethanol's temperature is further reduced below -70C/-94F undesirable plant waxes and chlorophyll are rendered almost completely insoluble, resulting in a significant reduction of both of these undesirables during an ethanol extraction. Ethanols' selectivity at these extremely low temperatures is more of a physical mechanism than a chemical mechanism. At these low temperatures, there is such a drastic decrease in undesirable plant wax and chlorophyll solubility due to the increased viscosity of the ethanol making it much more "honey-like" and physically less able to permeate plant cell walls where these undesirable compounds reside.
The permeability of the biomass cell walls can also be attributed to the mill size of the biomass material. If the plant material is ground too finely, the cell walls are opened, allowing easy access for ethanol to dissolve undesirable plant waxes and chlorophyll. While shredding or hand-breaking biomass can reduce exposure beyond the plant's cell walls by leaving most cells closed. The extraction of plant waxes and specifically chlorophyll, are commonly unavoidable to some extent. Regardless, when cold ethanol extraction is performed properly it significantly limits the co-extraction of these undesirables from biomass material to the extent where only a small amount of plant waxes are extracted, making a winterization process unnecessary for further processing into a distillate.

Cryogenic 45L Centrifuge Extractor Kit By BVV
Commonly, ethanol is chilled to a range of -30C to -40C to increase the selectivity of the extraction and limit the co-extraction of impurities. While ethanol can be chilled even colder to further increase its selectivity, this becomes rather costly and can be less than cost-effective due to the energy requirements necessary to chill large volumes of ethanol past -40C/-40F. Typical methods of chilling ethanol solvent include a low-temperature explosion-proof freezer or a high-powered ethanol chiller that chills the ethanol prior to injection into the centrifuge.
Conversely, room temperature or "warm" (15-30C/59-86F) ethanol can be utilized to perform the initial saturation. When utilizing room temperature or "warm" ethanol for botanical extraction, the ethanol has greater solubility and saturation capacity allowing the solvent to effectively dissolve target compounds from the plant matter. While this strategy allows for the target compounds to be more effectively dissolved from the plant matter, it also allows for undesirable botanical compounds like color pigments and plant waxes to be more easily dissolved, resulting in a greater number of total impurities.
Typically a warm ethanol extraction is paired with post-processing in the form of winterization and media filtering to remove co-extracted plant waxes, and undesired pigments, creating a refined botanical extract referred to as winterized or refined crude oil. Warm ethanol extraction is typically better suited for large-scale processors because the cost of chilling large volumes of ethanol is greater than the cost of dewaxing or color remediating the extracted crude oil.
Regardless of the extraction temperature strategy, once the solvent has reached the desired extraction temperature, the solvent is then injected into the centrifuge, saturating the biomass material. When it comes to ethanol saturation, due to ethanols polar nature, it is magnetically attracted to other polar compounds, which explain ethanols propensity to extract undesirables from plant matter so rapidly. Chlorophyll extraction is commonly unavoidable during an ethanol extraction, even with short retention times. This leads most ethanol extractors to opt for a longer retention time strategy to ensure target compounds are effectively extracted from the biomass material.
Additionally, Alcohols are amphiphilic, meaning they are both polar and nonpolar at the same time. The OH groups of ethanol are capable of hydrogen bonding which is the strongest of the weak intermolecular bonds. This is one of the reasons why alcohols are so efficient even with very short retention times at low temperatures, but also why they dissolve a lot of undesirables when contact time or temperatures are high. For these reasons, typical short retention time quick to wash methods are not as effective at limiting the co-extraction of impurities which leads most ethanol extractors to opt for a longer retention time between 3-10 minutes to ensure target compounds are effectively extracted.
Since quick wash saturation methods are not as effective at limiting impurities for ethanol extractions and ethanol has such a large saturation capacity, it is not uncommon for the same volume of ethanol to be reused for multiple saturations of fresh biomass. Reuse of saturated ethanol is commonly extended for 2-3 runs before an appreciable reduction of extraction efficiency is noticed. It is not uncommon for some extractors to opt to use the same volume of ethanol for up to 6 runs before opting to perform solvent recovery or winterization to get the most out of a given volume of ethanol. While the reuse of the same ethanol for multiple runs can reduce downtime between extractions, shorten the solvent recovery process, and reduce overall production costs, it does come at the cost of reduced extraction efficiency. Keep in mind this strategy is not as applicable with cold ethanol extraction due to the reduced saturation capacity at reduced temperatures and the potential for saturated ethanol to clog or gum up the chilling ethanol equipment.
Certified 160L Jacketed Stainless Steel Centrifuge with Explosion Proof Motor and Siemens Controller by BVV
Due to ethanol's high saturation capacity, ethanol saturation volume is typically approached by fully submerging the biomass in ethanol within a given volume rather than aiming for a specific solvent to biomass ratio. This is commonly achieved by closing the outlet to the extraction vessel and filling it to completely submerge the biomass in ethanol 1-2" above the plant matter to fully saturate biomass. Once the biomass has been completely submerged, the centrifuge is commonly agitated bidirectionally to achieve a complete saturation of the plant material for 3-10 minutes with rotational direction changes every 15-30 seconds.
Both retention time and agitation are important factors in allowing the solvent to fully dissolve the target compounds. Too little retention time or agitation can result in reduced yield of target compounds, while too long retention time/agitation can allow for more undesirable compounds like chlorophyll and plant waxes to be dissolved. Commonly ethanol extractors opt for longer retention times to ensure target compounds are fully dissolved and rely on post-processing methods like winterization and distillation to remove impurities and reach the desired level of purity.
Extended retention times are great if looking to extract all that’s possible from the plant matter. When this strategy is paired with separate post-processing in the form of winterization and distillation, it can result in both high yield and purity. Conversely, a short retention time minimizes the time the solvent has to co-extract undesirables. When a quick wash/short retention time strategy is utilized in conjunction with chilled solvent, it can result in a concentrate with very low levels of impurities that can be easily removed through inline filtration prior to solvent recovery. While utilizing both a short retention time and chilled solvent typically results in a slightly lower extraction yield, this is commonly unavoidable without extending saturation times and increasing the co-extraction of impurities.
Explosion-Proof Touch Screen Centrifuge Controller by BVV
To get the most out of the retention time, commonly, the centrifuge is spun at low RPM between 100-200 RPMs with bidirectional rotation every 15-30 seconds to ensure the biomass is fully saturated. After the desired agitation time is complete, the solution is then drained from the centrifuge, and the biomass is spun at high rpm, applying centrifugal force to recapture the residual ethanol still trapped within the biomass. This is typically done for 5-10 minutes in the same direction at increasing rpm rates in order to recapture as much ethanol from the biomass as possible.
After the initial saturation and solvent recapturing is complete and the entirety of the botanical extract-rich ethanol solution has been drained into the collection vessel the ethanol extraction process is now complete. The resulting solution can either be refined through the process of winterization to precipitate out and filter the plant waxes before the solvent recovery process or winterization can be skipped and the process of solvent recovery can be performed by heating the solution to the boiling point of ethanol 173F/78C to evaporate the ethanol solvent from the botanical extract solution. Both processes result in a crude botanical extract that can be further refined via distillation, with a winterized or cold-extracted extract being more easily distilled. Both the process of winterization and solvent recovery will be covered in-depth in the next chapters. Now that we have covered the ethanol extraction process, let's go over a standard ethanol extraction preparation and processing procedure.
Falling Film Evaporator By BVV
Preparation for ethanol extraction starts with the milling of the biomass material. Proper mill size is paramount to success during the extraction process. An improper mill size and poor packing can lead to an unbalanced load and dry spots during extraction. An unbalanced load on the centrifuge causes excessive harmonic vibration and potential damage to the system. Additionally, If the biomass is milled too finely, the cellular walls may rupture, which may lead to an excessive amount of undesirable compounds being dissolved into the extract solution. To minimize the pickup of undesirable chlorophyll and plant waxes and ensure even distribution, proper milling is key.
Biomass material can come in a variety of different types. Packing highly variable biomass types into the same centrifuge bag can result in an unbalanced load during extraction. A homogenous volume of biomass will perform best during extraction; buds or flowers should be coarsely milled to an ideal mill size between 1/8" -1/4". Un-milled biomass should not be used. Full buds require longer residence time for extraction, and stems can cause damage to the centrifuge bags. Biomass that is too finely milled should not be used either. Too fine of a mill can cause an uneven distribution of alcohol which can cause excessive harmonic vibration and leave dry spots. A coarse mill is best for biomass with a recommended mill size between 1/8"-1/4".
During packing of centrifuge bags it is ideal to use cylindrical support like a bucket or an appropriately sized diameter ducting to keep the bag uniform. As the biomass is loaded into the bag, ensure the biomass is evenly distributed by pushing down on the biomass every few handfuls to promote an even distribution. Always ensure centrifuge bags are filled completely and evenly to promote a balanced spin. Partially filled bags can lead to imbalance during extractionand excessive harmonic vibration.
45L Centrifuge Bag By BVV
Once the centrifuge bag has been appropriately packed, place the bag into the centrifuge basket and push down on the material to ensure a proper seat and even distribution of biomass material within the centrifuge basket. Close the lid and lock all clamps into place. Once the lid has been secured into place, and the extraction solvent has reached the desired temperature, ensure the output of the centrifuge is closed before proceeding to inject solvent into the grounded centrifuge from a bonded container. Depending on the centrifuge design, use either pressure or vacuum to inject solvent into the system. If utilizing pressure to inject into the system, no more than 15 PSI of pressure should be utilized to inject into the system. Ensure the vent valve of the centrifuge is open to ensure the centrifuge does not become pressurized and begin filling the centrifuge. As the solvent enters the vessel saturating the loaded biomass, allow the centrifuge to fill 1" to 2" above the centrifuge bag to ensure full saturation without overfilling the centrifuge.
Once the centrifuge has been loaded with the desired amount of ethanol, close the vent valve before proceeding to agitate the solution at 125 RPM for 3-10 minutes, changing rotational direction every 15-30 seconds to ensure the biomass material is saturated fully. Once the agitation cycle is complete, open the vent valve located on the lid of the centrifuge and open the drain valve allowing the system to drain into the collection vessel.
Once the system has been completely drained and only a small volume of liquid remains, proceed to spin dry the biomass at high rpm utilizing the following procedure. Two minutes at 400 rpm, 1.5 minutes at 600 rpm, 1 minute at 800 rpm, and 2 minutes at 1200 rpm. By utilizing an incremental increase in rpm as the solvent is evacuated from the biomass, it ensures harmonic vibration is reduced and ethanol is recovered from the biomass with great efficiency.
Once the spin-dry sequence has completed and the rotation stops it is now safe to unlatch the lid, remove the centrifuge bag, and discard the biomass. Once the bag has been removed, wipe down the system of any residual particulate or biomass using a clean rag and alcohol as a cleaning agent. The ethanol extraction process is now complete; the resulting botanical extract solution can now be winterized and filtered if necessary, and a solvent recovery process can be initiated.
280L Jacketed Stainless Steel Centrifuge & Controller By BVV

ETHANOL EXTRACTION STANDARD OPERATING PROCEDURE
Purpose
The purpose of this procedure is to provide detailed instructions for centrifugal ethanol extraction.
Scope
This procedure applies to all lab technicians tasked with centrifugal ethanol extraction.
Definitions/Acronyms
Personal Protection Equipment (PPE) Items worn to protect employees from exposure to hazardous materials and prevention of injury.
Safety Data Sheet (SDS) Provides valuable information on chemicals, describing the hazards the chemical presents and giving information on handling, storage, and emergency measures in case of an accident.
Safety
SDS Sheets: Ethanol SDS: 710 Extraction solvent SDS
PPE: The following should be worn by all lab personnel during this refinement procedure:
Protective eyewear
Lab coat
Nitrile Gloves (Low-temperature gloves as needed)
Respirator
5. Hazard Identification
Preparation and Use:
Target compounds will be extracted from biomass material using ethanol as the solvent.
Concentration- Use a 200 proof Ethanol or 710 extraction solvent (BVV)
Quantity- Volume of the centrifuge capacity of ethanol or 710 extraction solvent + centrifuge bag capacity of milled biomass
Frequency- An initial volume of solvent is used, no more is added.
Location- Inside of a grounded explosion-proof, stainless steel extraction centrifuge inside a C1D2 environment.
Potential Hazards and Risks
See ethanol or 710 extraction solvent SDS for detailed risks.
6. Preparation
7. Procedure